Gene for coding recombinant NlpC/P60 endopeptidase protein and application thereof
A technology of P60 and endopeptidase, applied to the gene encoding recombinant NlpC/P60 endopeptidase protein and its application field, can solve the problems of limited success rate and achieve the effect of low cost, high purity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
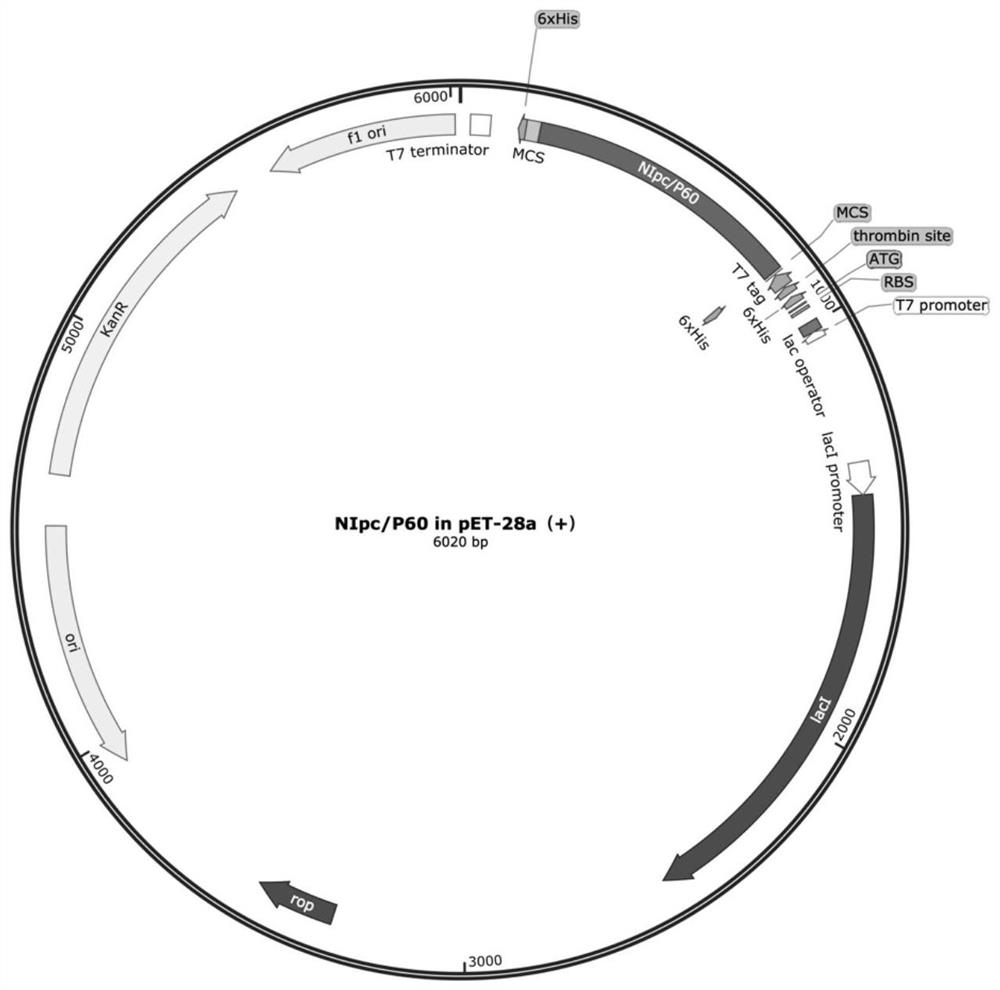
[0052] Example 1 Construction of NlpC / P60 endopeptidase protein particle NlpC / P60 in pET-28a(+)
[0053] (1) According to the amino acid sequence of NlpC / P60 endopeptidase protein, optimize its nucleotide sequence, obtain the nucleotide sequence of the optimized NlpC / P60 endopeptidase protein, wherein, NlpC / P60 endopeptidase protein The original nucleotide sequence and the nucleotide sequence of the optimized NlpC / P60 endopeptidase protein are as follows:
[0054] Original nucleotide sequence of NlpC / P60 endopeptidase protein:
[0055] ATGTGGTTCAAGGGGGTACTCACGATCTCTGCCCTACTACTAATCCTGTCAGGGTGTTCAACCAAATCCATAGACCCATACGGCGAACAGAATTCCTATGACAACATTGGCTCCAAGTACCAAAAGAATGATACCACGAGTGGATATAACTTCAATTACAACCAAGCTGCCGACGAGTTTTATAACATAAATCTCTCCAAATATCTTAACAAAAAGTTAGGTAATGACTGCAGTGGATTCGTGTCTTTAGTTAATGAGGATTCCAAATCGTTATATTTCGACGAAAATATTGTCAATAATTTCTACGACAAAAACGGCCGTAAGAGCCAAGCCATCTTTAACCTCTACAAGTCACAGAACAAGATCTCATATACCGATCCTAAACCTGGTGATCTGGTGTTCTTCAACAACACGACATCTCGCACAAACAAGTCTAAGAATAAAGCCAT...
Embodiment 2
[0062] Induced expression of embodiment 2 recombinant NlpC / P60 endopeptidase protein
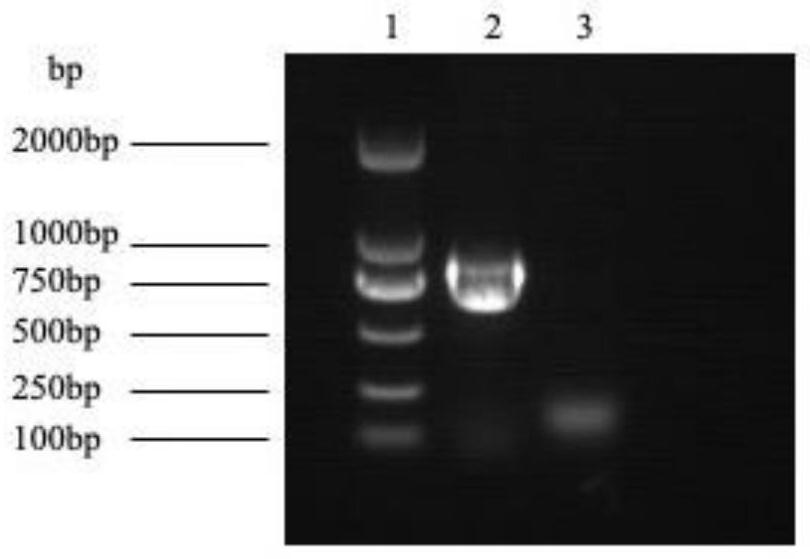
[0063](1) The recombinant expression plasmid NlpC / P60in pET-28a(+) (using NlpC / P60'in pET-28a(+) as a control) preserved in Example 1 for expressing recombinant NlpC / P60 endopeptidase protein was routinely Methods Transformed into the recipient Escherichia coli BL21(DE3), the specific method was as follows: 25 μg of recombinant expression plasmid and 100 μL of BL21(DE3) competent cells were mixed, placed on ice for 30 minutes, heat-shocked in a water bath at 42°C for 90 seconds; then added 1ml of LB Broth culture medium, at 37 ℃, resuscitated and activated on a shaking table at 200rpm for 1h, centrifuged at 3000rpm for 1min, discarded 90% of the supernatant, and coated the bacterial solution with LB containing kanamycin (containing 30mg / L of kanamycin (Kan)) plate for resuscitation and activation, cultured at 37°C for 16 hours; pick a single colony and use T7 primers for colony PCR confirm...
Embodiment 3
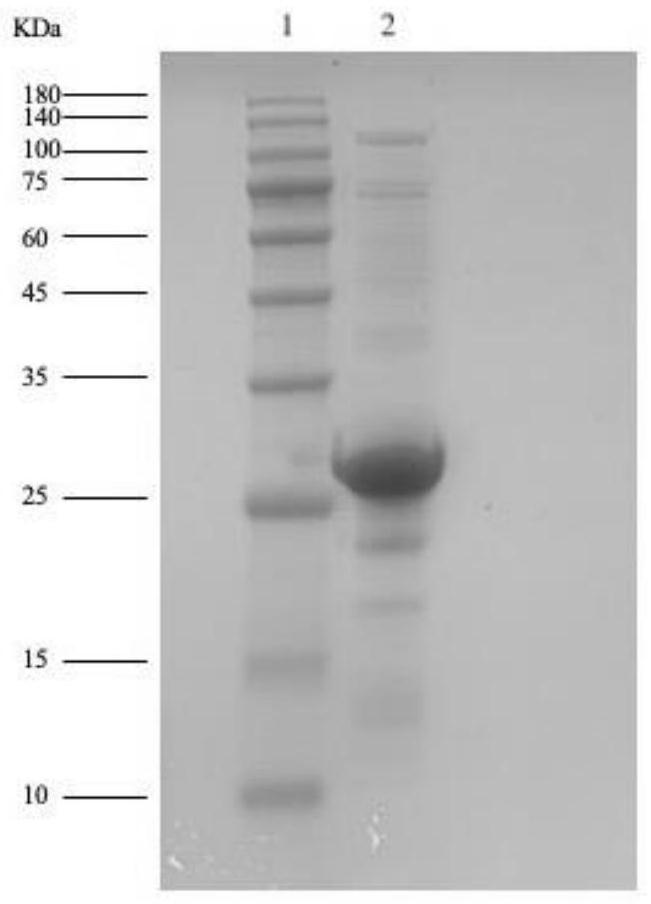
[0074] Embodiment 3 Purification, dialysis and sterilization of recombinant NlpC / P60 endopeptidase protein
[0075] (1) Equilibrium column: first use ddH 2 O removes ethanol from the nickel column, ddH 2 O is greater than 5 column volumes, then use Lysisbuffer for column equilibration.
[0076] (2) Sample loading: Slowly inject the supernatant obtained in step (4) of Example 2 into the nickel column.
[0077] (3) Elution: Use 500 μl Elution buffer to elute the column, use a 1.5mL ep tube to collect the liquid dripping from the nickel column, and elute 4 times to obtain 4 tubes of purified protein.
[0078] (4) Dialysis: Take the 2nd and 3rd tube proteins in step (3), put them into a 3000Da protein dialysis bag, add 1L PBS (pH=7.4), put them in a 4°C refrigerator, and dialyze the protein samples until the next day.
[0079] (5) Sterilization: Take the dialyzed protein in a 1.5ml ep tube, add glycerol with a volume fraction of 20%, centrifuge at 12000g for 5min, take the supe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com