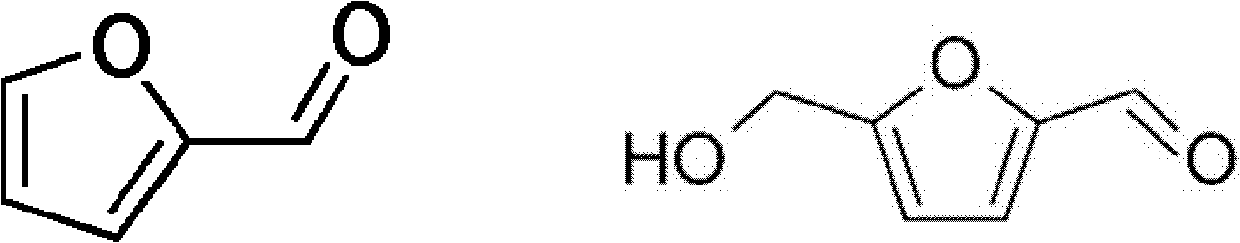Preparation of furfural compounds, and mixture for preparing the same
一种混合溶液、化合物的技术,应用在有机化学等方向,能够解决不易分离纯化、不易控制副反应、反应效率低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
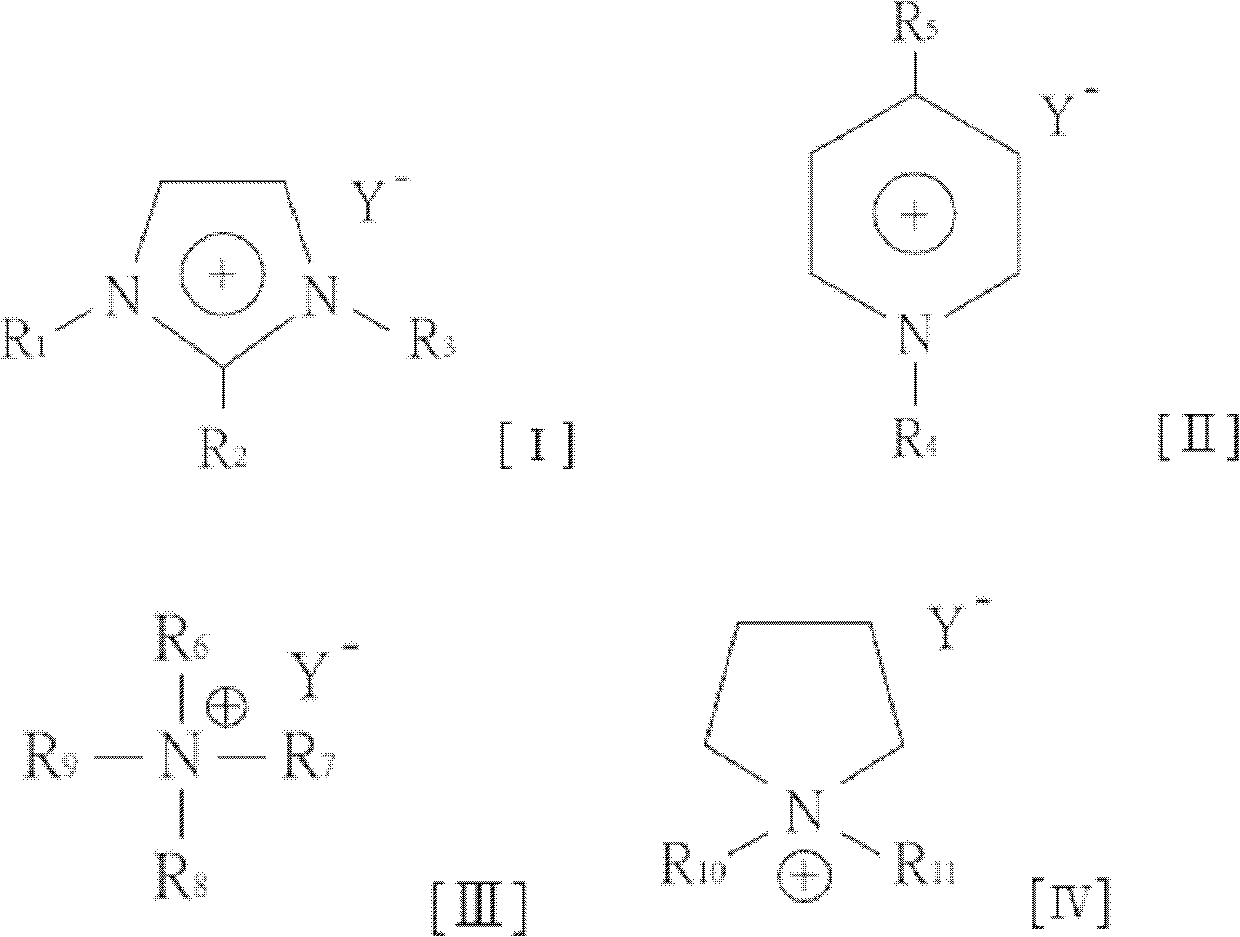
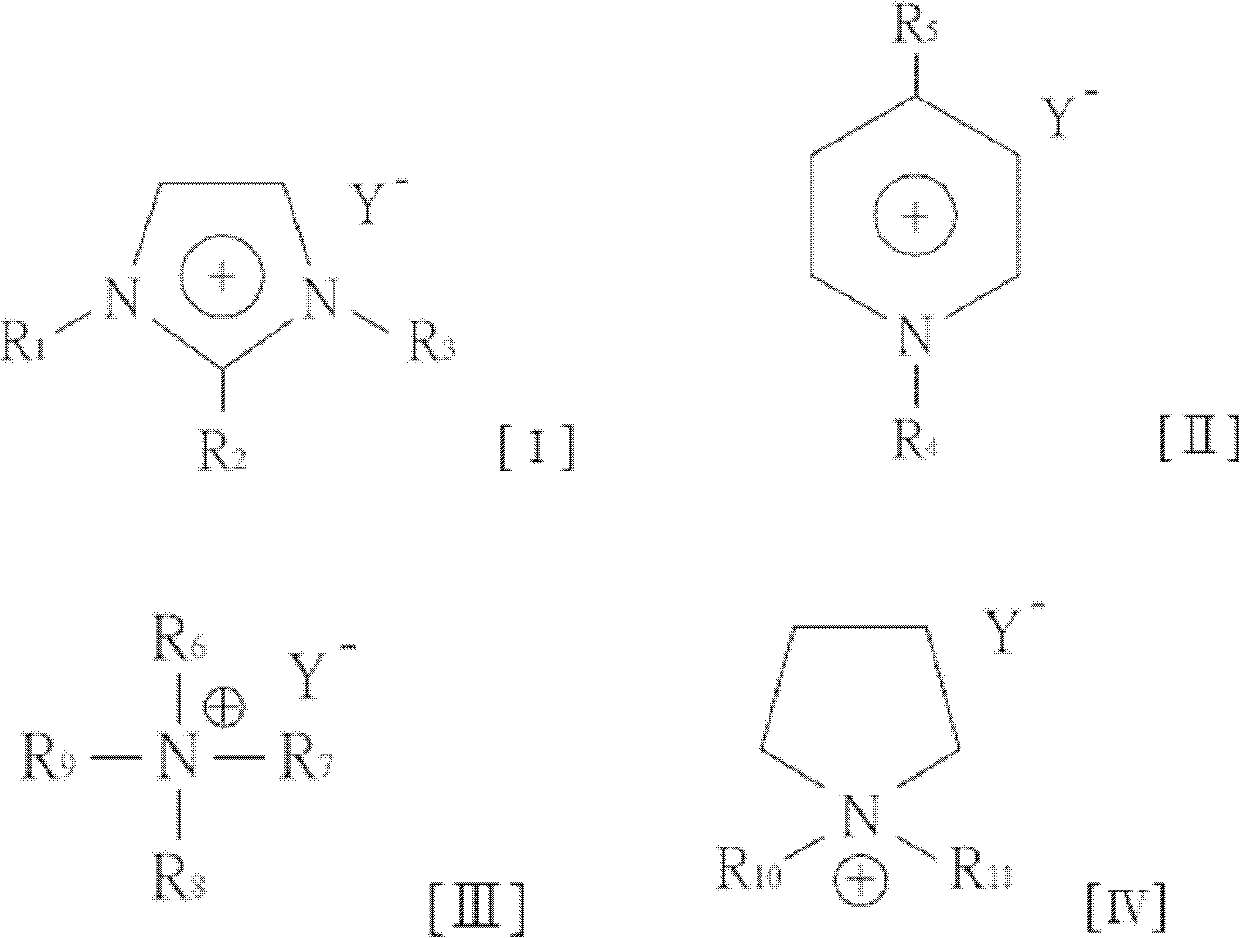
[0011] The embodiment proposes a preparation method of furfural compounds and a mixed solution thereof. In one embodiment, a method for preparing furfural compounds (taking 5-hydroxymethylfurfural or furfural as an example) includes the following steps. An organic ammonium salt is mixed with a hydroxyl-containing organic solvent to form a solution composition. Next, a carbohydrate is mixed into the solution composition to form a mixed solution. Heating the mixed solution to a reaction temperature converts the carbohydrate into furfural compounds such as 5-hydroxymethylfurfural (HMF) or furfural. The molar ratio of the organic ammonium salt to the hydroxyl group-containing organic solvent is 1 to 9, or 2.5 to 4; the proportion of carbohydrates in the mixed solution is 5 to 20 wt%, or 10 wt%.
[0012] In one embodiment, the organic ammonium salt may be a chain organic ammonium salt or a cyclic organic ammonium salt.
[0013] In one embodiment, the chain organic ammonium salt ...
Embodiment 1
[0028] Choline chloride is mixed with different hydroxyl-containing organic solvents, and in CrCl 2 Catalyst converts glucose to HMF
[0029] In embodiment one, select choline chloride as organic ammonium salt, CrCl 2 as catalyst, and glucose as carbohydrate. Mix choline chloride with each hydroxyl-containing organic solvent according to the required molar ratio and heat to 100°C (heating temperature is less than 120°C) to form a solution composition. After mixing glucose, add CrCl relative to the number of moles of glucose 2 Catalyst, reaction at 100°C (conversion temperature, eg, 100°C-120°C) into HMF product. Table 2 lists each experimental condition and the experimental result of HMF productive rate among the embodiment one.
[0030]Among them, embodiment 1-1 selects 1,3-propanediol as the hydroxyl-containing organic solvent, embodiment 1-2 selects 1,4-butanediol as the hydroxyl-containing organic solvent, and embodiment 1-3 selects diethylene glycol as the hydroxyl-co...
Embodiment 2
[0034] choline chloride and diethylene glycol solvent are mixed according to different ratios or change glucose concentration, and in CrCl 2 Catalyst converts glucose to HMF
[0035] In embodiment two, select choline chloride as organic ammonium salt, diethylene glycol is the hydroxyl organic solvent, CrCl 2 as the catalyst, and glucose as the carbohydrate. Mix choline chloride to diethylene glycol solvent according to the required molar ratio and heat to 100°C to form a solution composition. After mixing glucose, add 6 mol% (relative to the number of moles of glucose) CrCl 2 Catalyst, react at a temperature of 100°C to convert to HMF product.
[0036] Table 3 lists each experimental condition and the experimental result of HMF productive rate among the embodiment two. Among them, in Example 2-1 to Example 2-3, the yields of HMF were 57%, 61% and 56% respectively.
[0037] table 3
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com