Method for promoting osteoblast differentiation by using Runx2 and Osterix and application thereof
An osteoblast differentiation and protein technology, which is applied in the use of Runx2 and Osterix to promote osteoblast differentiation and its application fields, can solve the problems of ineffective use of osteoblast differentiation and bone formation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Plasmid Construction and Cell Culture
[0047] Cell culture
[0048] Plat-E cells (Cell Biolabs Inc.) were cultured in a medium containing 10% fetal bovine serum (Hyclone), 1 μg / ml puromycin (puromycin, Sigma), 10 μg / ml blasticin (brasticidine, Sigma), 100 U / ml Penicillin (Sigma), 100 μg / ml streptomycin (Sigma) in DMDM medium (Gibco) and adhered to 6-well plates coated with rat tail type I collagen. C3H10T1 / 2 cell lines (ATCC, CCL-226) and other cell lines obtained from C3H10T1 / 2 cells were cultured in DMDM medium containing 10% fetal bovine serum, 100 U / ml penicillin, and 100 μg / ml streptomycin middle. The above cells were cultured at 37°C, 5% CO 2 in the incubator.
[0049] Plasmid construction
[0050] Insert the cDNA of FLAG-tagged full-length Runx2 (SEQ.ID.No.2, derived from mouse) and FLAG-tagged full-length Osterix (SEQ.ID.No.4, derived from mouse) into pMXs / neo Vector (Cell Biolabs Inc), or insert FLAG-tagged full-length Osterix (SEQ.ID.No....
Embodiment 2
[0052] Example 2: Establishment of C3H10T1 / 2 cell lines stably expressing Runx2 or Osterix respectively and stably expressing Runx2 and Osterix simultaneously
[0053] This example was accomplished by cell transfection, retrovirus infection and immunoblotting.
[0054] Specifically, pMXs / neo (as a negative control), and the Runx2-pMXs / neo constructed in Example 1, and the Osterix-pMXs / neo plasmids were transformed into Plat-E cells with Fugene 6 transfection reagent (Promega) . After 72 hours, the supernatant of the medium (DMEM medium containing 10% fetal bovine serum) contained the retrovirus. After filtering through a 0.45 μm filter membrane (Milipore), the filtrate was mixed with 5 mg / ml polybrene (polybrene, Sigma) at a ratio of 1000:1 and added to the C3H10T1 / 2 cells adhered one day in advance. After 6 hours, fresh medium was changed and culture was continued for 24 hours. The cells were screened with G418 to obtain the cells infected by the retrovirus, and the uninfe...
Embodiment 3
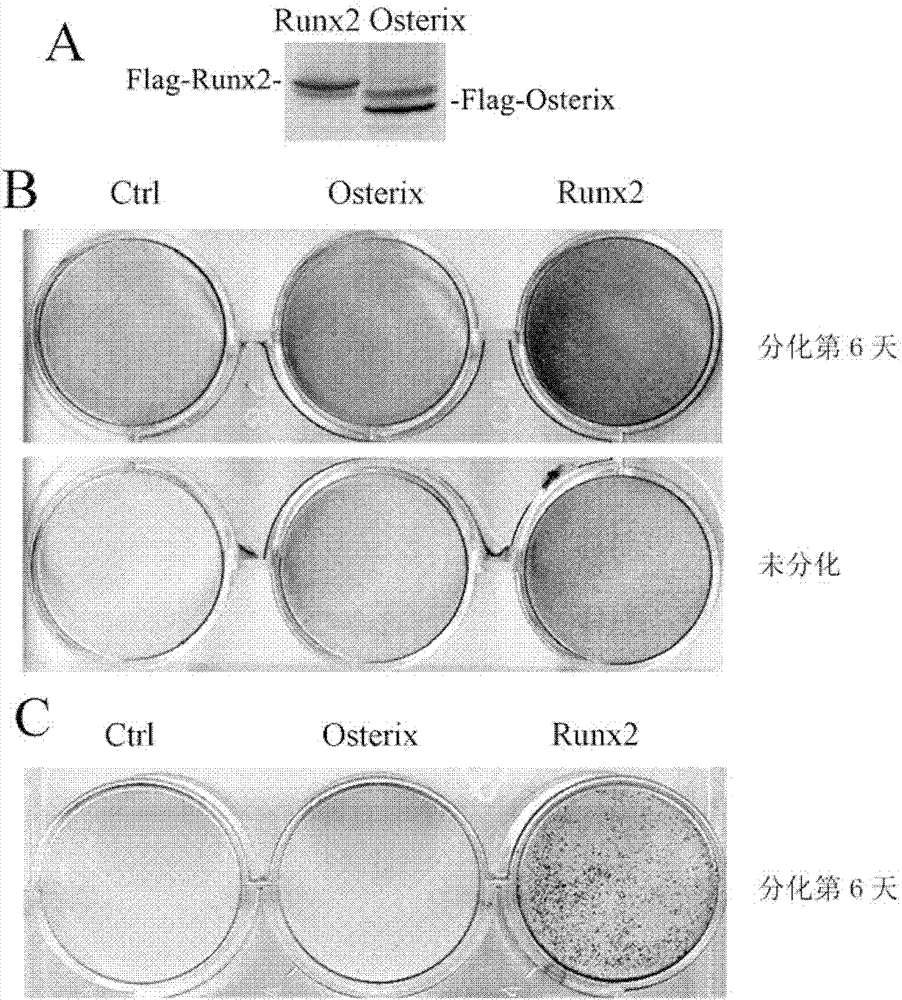
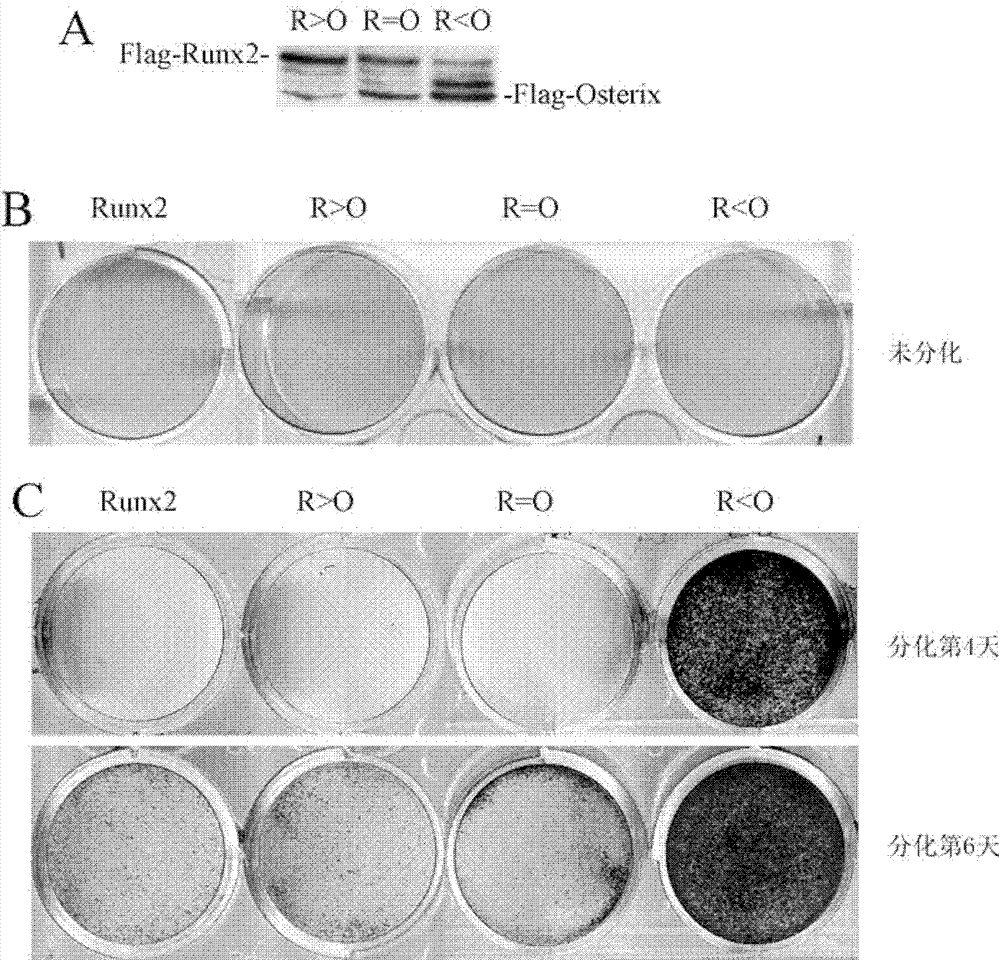
[0057] Example 3: The ability of Osterix to induce osteoblast differentiation is weaker than that of Runx2
[0058] The Ctrl, Runx2 and Osterix cells obtained in Example 2 were differentiated in osteoblast differentiation medium (adding 100 μg / ml ascorbic acid (Sigma) and 5 mM β-glycerophosphate (β-glycerophosphate, Sigma) to normal medium) for 6 Alkaline phosphatase activity staining and von Kossa staining were performed two days later. It was found that the ability of Osterix to induce alkaline phosphatase activity level and calcification level was significantly weaker than that of Runx2. See results figure 1 B and figure 1 c.
[0059] The Ctrl, Runx2 and Osterix cells obtained in Example 2 were also cultured in normal medium until confluent and stained for alkaline phosphatase activity. The results still show that Osterix is weaker than Runx2 in inducing the level of alkaline phosphatase activity. See results figure 1 b.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com