Tetrandrine gallate and drug composition, preparation method and applications thereof
A technology of tetrandrine and stearate, which is applied in the directions of drug combination, carboxylate preparation, pharmaceutical formulation, etc., can solve the problems of complicated process and insufficient compound activity, and achieves the effect of broad application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
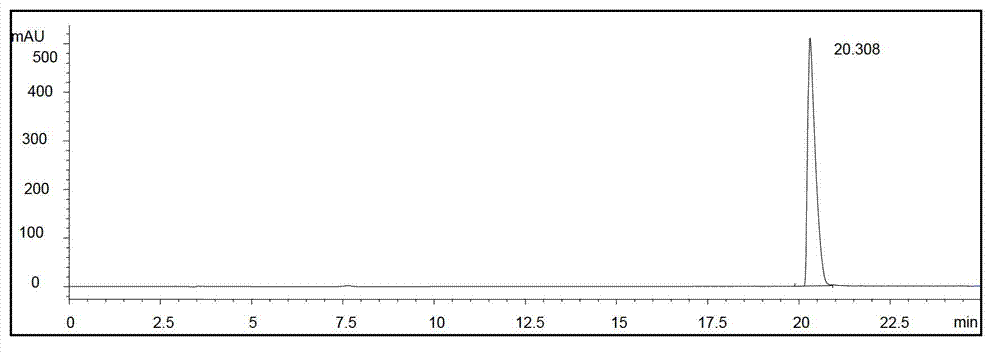
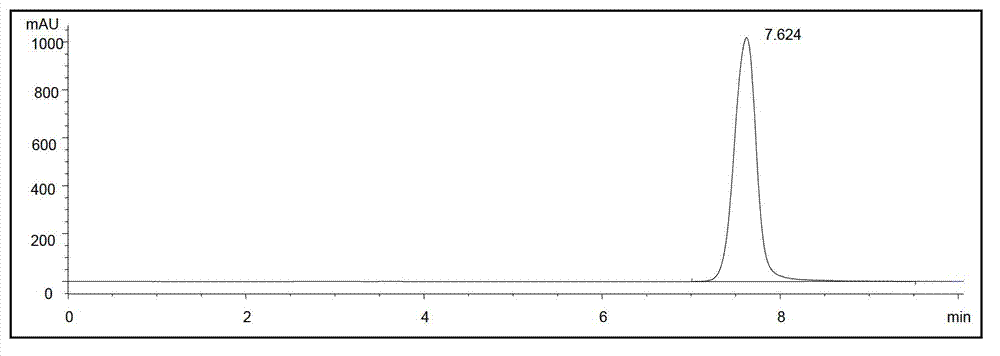
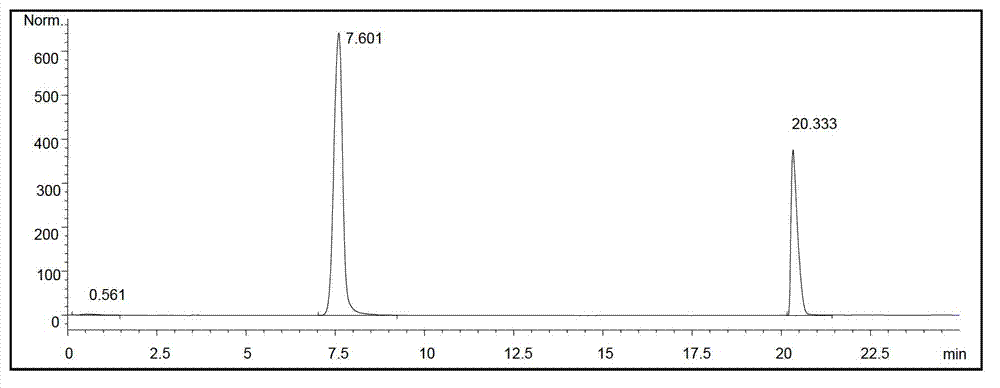
Image
Examples
Embodiment 1-5
[0051] The preparation of embodiment 1-5 tetrandrine gallate
[0052] The raw material formula and reaction parameters for synthesizing tetrandrine gallate (Formula I) are shown in Table 1:
Embodiment 1
[0055] Example 1. Synthesis of Tetrandrine Gallate (Formula I)
[0056] With reference to the aforementioned synthetic route represented by the structural formula and the raw material formula and reaction conditions shown in Table 1, the synthetic method of tetrandrine gallate (formula I) of the present invention is as follows: room temperature, dark, tetrandrine (10g , 0.016mol) into 600mL of absolute ethanol, stirred, white turbidity, added dropwise gallic acid (6.01g, 0.035mol) dissolved in 50mL of absolute ethanol, after the addition was completed, the temperature was controlled at about 30°C, stirred for 24 hours, concentrated under reduced pressure Evaporate about 500 mL of ethanol, stir at room temperature for 2 h, filter with suction, wash with ethanol, and dry to obtain a milky white solid; concentrate the filtrate, filter with suction, wash with ethanol, dry, and combine the filter cakes to obtain a total of 14.88 g of a milky white solid, with a yield of 95%. The te...
Embodiment 2
[0059] Example 2, Synthesis of Tetrandrine Gallate (Formula I)
[0060] With reference to the aforementioned synthetic route represented by the structural formula and the raw material formula and reaction conditions shown in Table 1, the synthesis method of Tetrandrine gallate (Formula I) in Example 2 was the same as in Example 1, and 28.19 g of a milky white solid was obtained. Yield 90%. Test result m.p.176-177℃, 1 H-NMR (d 6 -DMSO, 300MHz) was the same as in Example 1, and was confirmed to be tetrandrine gallate (Formula I).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com