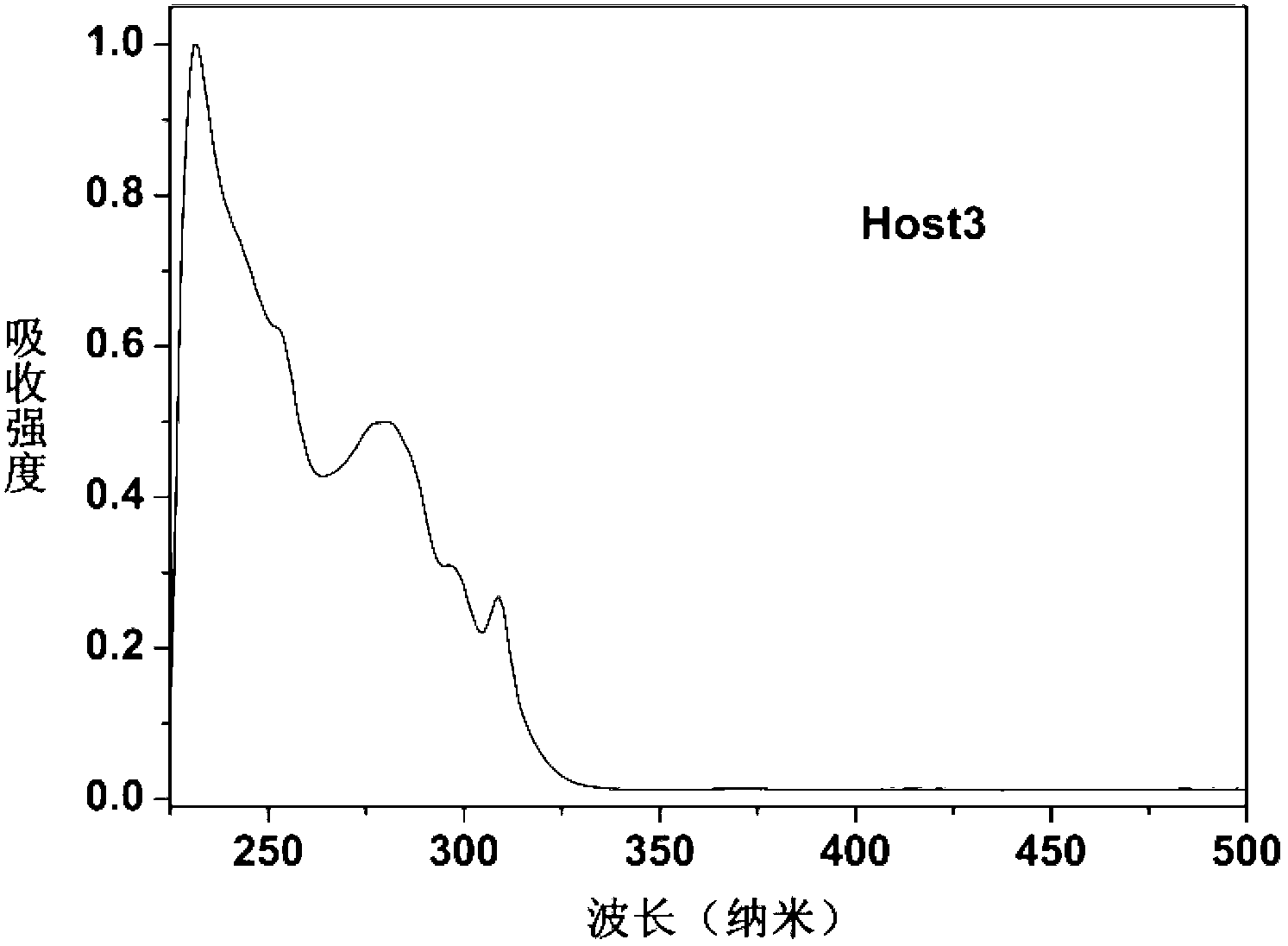
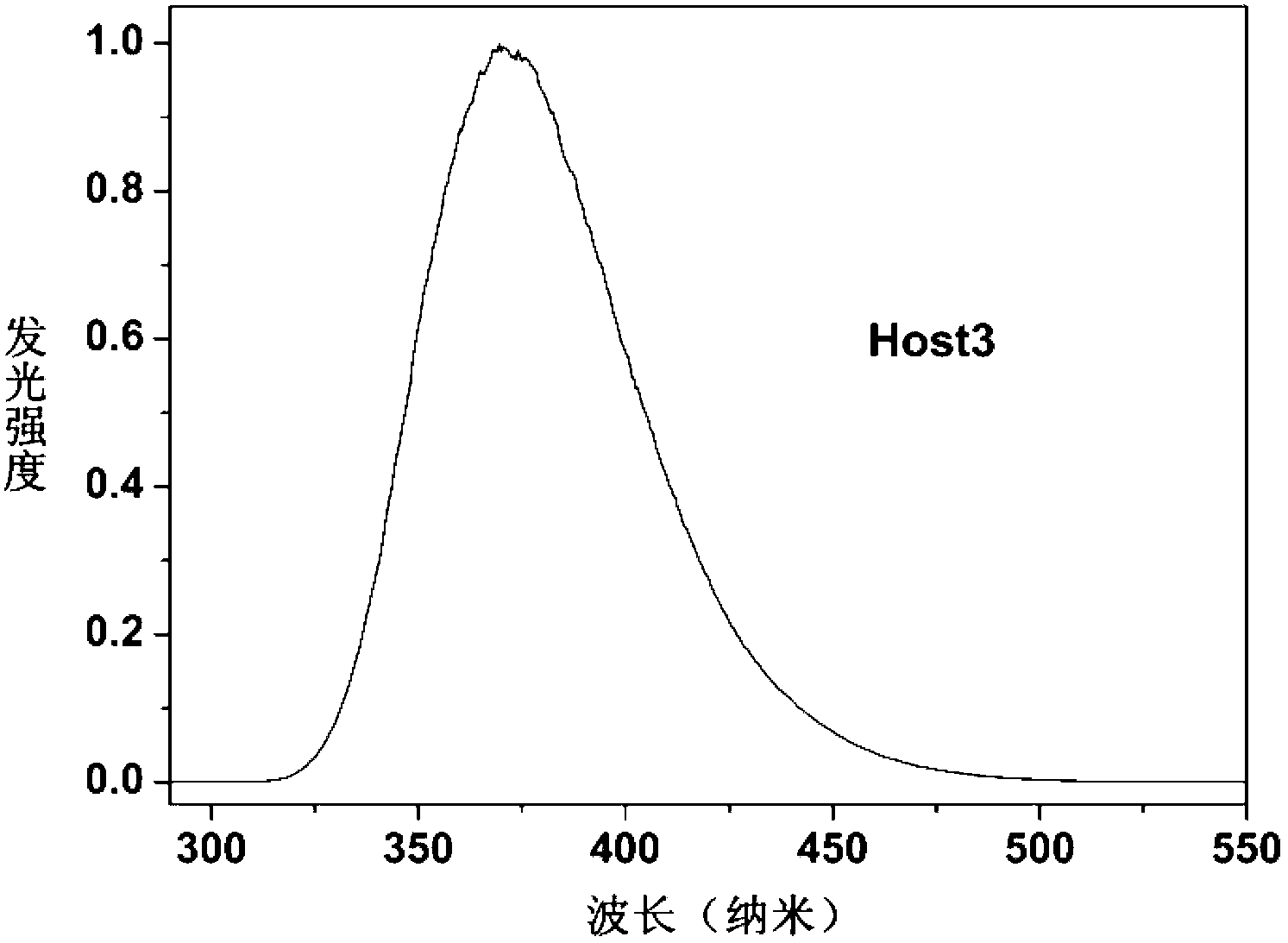
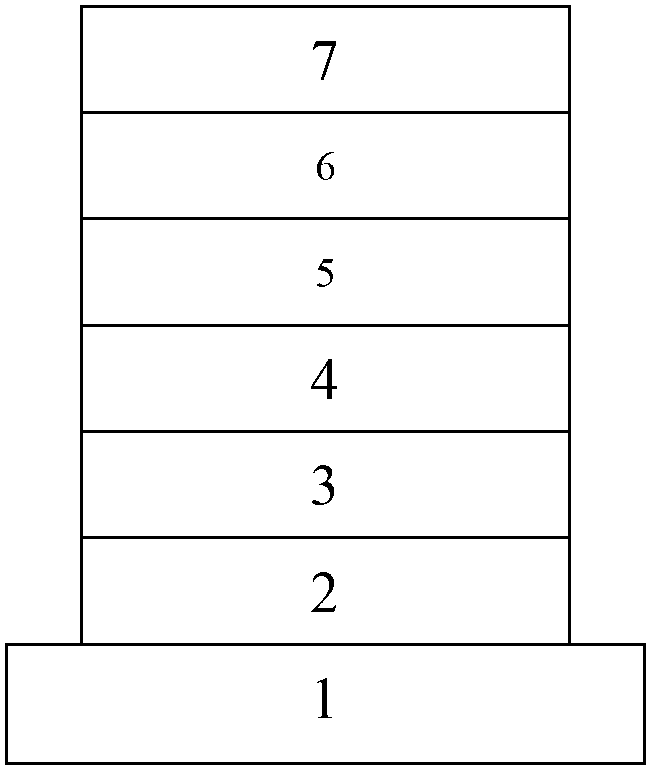
Dibenzo-heterocyclic spirobifluorene compound, preparation method thereof and organic electrophosphorescent device
A technology for phosphorescent devices and compounds, which is applied in the field of organic electrophosphorescent devices, dibenzoheterocyclic spirobifluorene compounds and their preparation, and can solve the problem of poor stability of luminescence, lack of hole transport ability, brightness and luminous power Can not meet the use requirements and other problems, to achieve the effect of good stability and good electroluminescent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The present invention also provides a preparation method of the compound shown in formula I, comprising:
[0054] A preparation method of a compound shown in formula 1, comprising:
[0055] Mixing the compound shown in formula IV or formula V with the compound shown in formula VI and alkali metal carbonate in an organic solvent, and a Suzuki coupling reaction occurs under the action of a catalyst to obtain a crude product;
[0056] The crude product was refluxed and extracted, and the extract was dried and subjected to silica gel column chromatography to obtain the compound shown in formula I;
[0057]
[0058] where X is O or S, X 1 is F, Cl, Br or I.
[0059] Owing to being mixed in the organic solvent by the compound shown in IV or formula V and the compound shown in formula VI and the carbonate of alkali metal, can find out by observing the structural formula of raw material compound that actually is between benzene ring and benzene ring Coupling reaction, bec...
Embodiment 1
[0077] Preparation of 4-2-spirobifluorenyl-dibenzofuran (abbreviated as Host1)
[0078]
[0079] Add 4.00 g of 2-bromospirobifluorene and 3.20 g of 4-dibenzofuran boronic acid into a 50 ml flask, add catalyst Pd(PPh 3 ) 4 650 mg, THF 42 mL, 2M K 2 CO 3 The solution was 14 ml, refluxed at 70°C for 24 hours under the protection of argon, extracted with dichloromethane after cooling, the organic layer was dried with anhydrous sodium sulfate and then spin-dried, passed through the column with dichloromethane / petroleum ether=1:5, and spin-dried 4.25 g of the target compound was obtained with a yield of 87.3%. 1 H NMR (400MHz, CDCl 3 )δ(ppm):8.00-8.07(m,2H),7.91(d,J=7.6Hz,2H),7.82-7.87(m,3H),7.36–7.46(m,6H),7.27–7.34(m ,2H),7.12–7.17(m,4H),6.84(d,J=7.6Hz,2H),6.79(d,J=7.6Hz,1H). 13 C NMR (100 MHz, CDCl 3 )δ (ppm): 155.9, 153.1, 149.4, 149.1, 148.7, 141.8, 141.5, 141.3, 135.9, 128.7, 127.9, 127.8, 127.7, 127.1, 126.6, 125.6, 124.7, 124.2, 124.6, 120.6, 1223.6 120.1, 120.0, ...
Embodiment 2
[0081] Preparation of 4-2-spirobifluorenyl-dibenzothiophene (abbreviated as Host2)
[0082]
[0083] Using a method similar to Example 1, 4-2-spirobifluorenyl-dibenzothiophene can be prepared using 2-bromospirobifluorene and 4-dibenzothiophene boronic acid as raw materials, with a yield of 92.2%. 1 H NMR (400MHz, CDCl 3 )δ(ppm):8.11(t,J=4.8Hz,1H),8.03(d,J=7.6Hz,1H),7.98(d,J=8.0Hz,1H),7.90(d,J=7.6Hz ,1H),7.80-7.85(m,3H),7.75(t,J=5.2Hz,1H),7.33-7.45(m,6H),7.32(d,J=7.6Hz,1H),7.14(t, J=7.6Hz,3H),7.05(s,1H),6.85(d,J=7.6Hz,2H),6.78(d,J=7.6Hz,1H). 13 C NMR (100MHz, CDCl 3 )δ (ppm): 149.6, 149.1, 148.5, 141.8, 141.6, 141.3, 140.1, 139.4, 138.5, 136.8, 136.1, 135.6, 128.0, 127.8, 127.7, 126.7, 126.6, 124.9, 124.1, 122.4 120.3,120.1,120.0,66.0.MS(EI):m / z 498.08(M + ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com