Method of synthesis of 2-picoline through 5-ketohexanenitrile
A technology of methyl pyridine and base capronitrile, which is applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, organic chemistry, etc., can solve the problems of low purity, unstable acetylene, and explosive polymerization. , to achieve the effect of strong operability, safe operation and reasonable design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
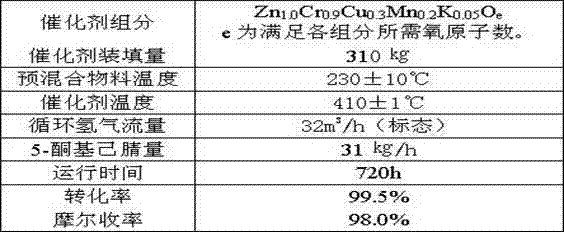
[0021] Example 1, a method for synthesizing 2-picoline from 5-ketocapronitrile, the method uses 5-ketocapronitrile (acetylbutyronitrile) as raw material, and in the presence of hydrogen, a granular catalyst is packed in a fixed bed , at a temperature of 380°C and a pressure of 0.02MPa, one-step synthesis of 2-picoline; the load of 5-ketocapronitrile is 40g / liter catalyst L·h, and the standard hydrogen circulation in the tail gas is 0.08m 3 / liter of catalyst L h;
[0022] Each active component of the granular catalyst is counted as:
[0023] Zn 1.0 Cr a Cu b mn c K d o e
[0024] The value of a ranges from 0.1 to 1.2;
[0025] The b value ranges from 0.1 to 0.6;
[0026] The value of c ranges from 0.05 to 0.5;
[0027] The range of d value is 0.01~0.08;
[0028] e is the sum of the oxygen required to satisfy the oxides of each element.
Embodiment 2
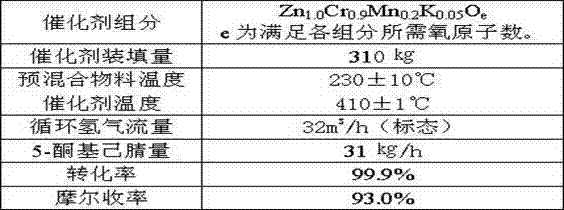
[0029] Example 2, a method for synthesizing 2-picoline from 5-ketocapronitrile, the method uses 5-ketocapronitrile (acetylbutyronitrile) as raw material, and in the presence of hydrogen, a granular catalyst is packed in a fixed bed , at a temperature of 420°C and a pressure of 0.1 MPa, 2-picoline was synthesized in one step; the load of 5-ketocapronitrile was 180 g / liter catalyst L·h, and the standard hydrogen circulation in the tail gas was 0.2 m 3 / liter of catalyst L h;
[0030] Each active component of the granular catalyst is counted as:
[0031] Zn 1.0 Cr a Cu b mn c K d o e
[0032] The value of a ranges from 0.1 to 1.2;
[0033] The b value ranges from 0.1 to 0.6;
[0034] The value of c ranges from 0.05 to 0.5;
[0035] The range of d value is 0.01~0.08;
[0036] e is the sum of the oxygen required to satisfy the oxides of each element.
Embodiment 3
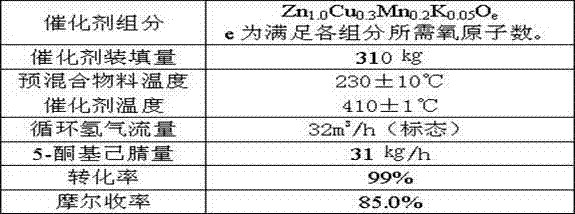
[0037] Example 3, a method for synthesizing 2-picoline from 5-ketocapronitrile, the method uses 5-ketocapronitrile (acetylbutyronitrile) as raw material, and in the presence of hydrogen, a granular catalyst is packed in a fixed bed , at a temperature of 400°C and a pressure of 0.06 MPa, one-step synthesis of 2-picoline; the load of 5-ketocapronitrile is 100 g / liter catalyst L·h, and the standard hydrogen circulation in the tail gas is 0.12 m 3 / liter of catalyst L h;
[0038] Each active component of the granular catalyst is counted as:
[0039] Zn 1.0 Cr a Cu b mn c K d o e
[0040] The value of a ranges from 0.1 to 1.2;
[0041] The b value ranges from 0.1 to 0.6;
[0042] The value of c ranges from 0.05 to 0.5;
[0043] The range of d value is 0.01~0.08;
[0044] e is the sum of the oxygen required to satisfy the oxides of each element.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com