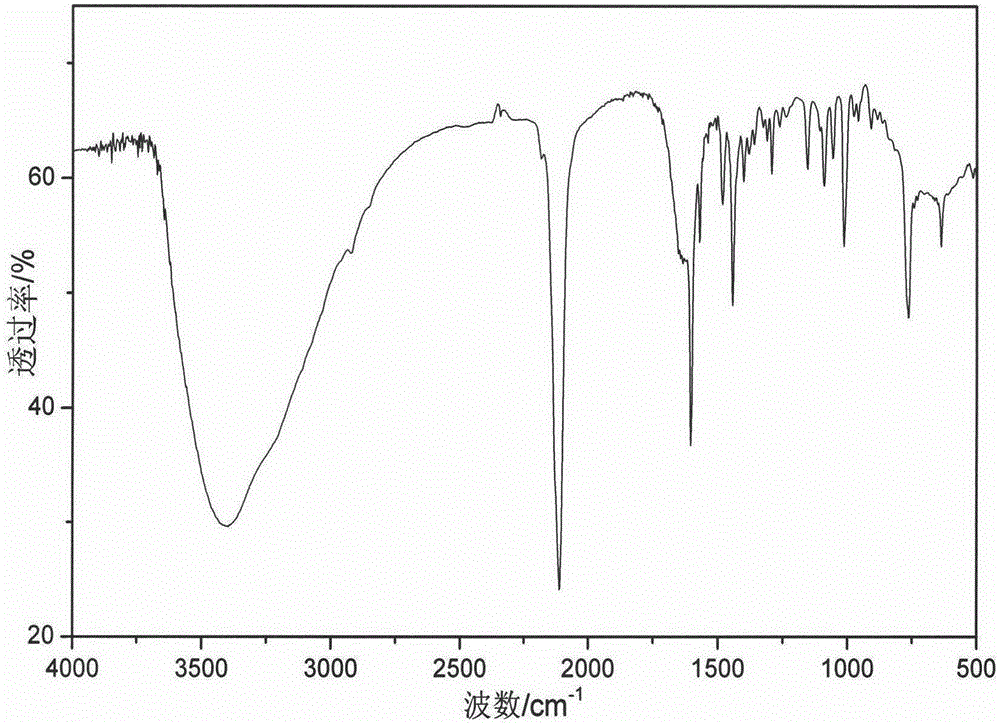
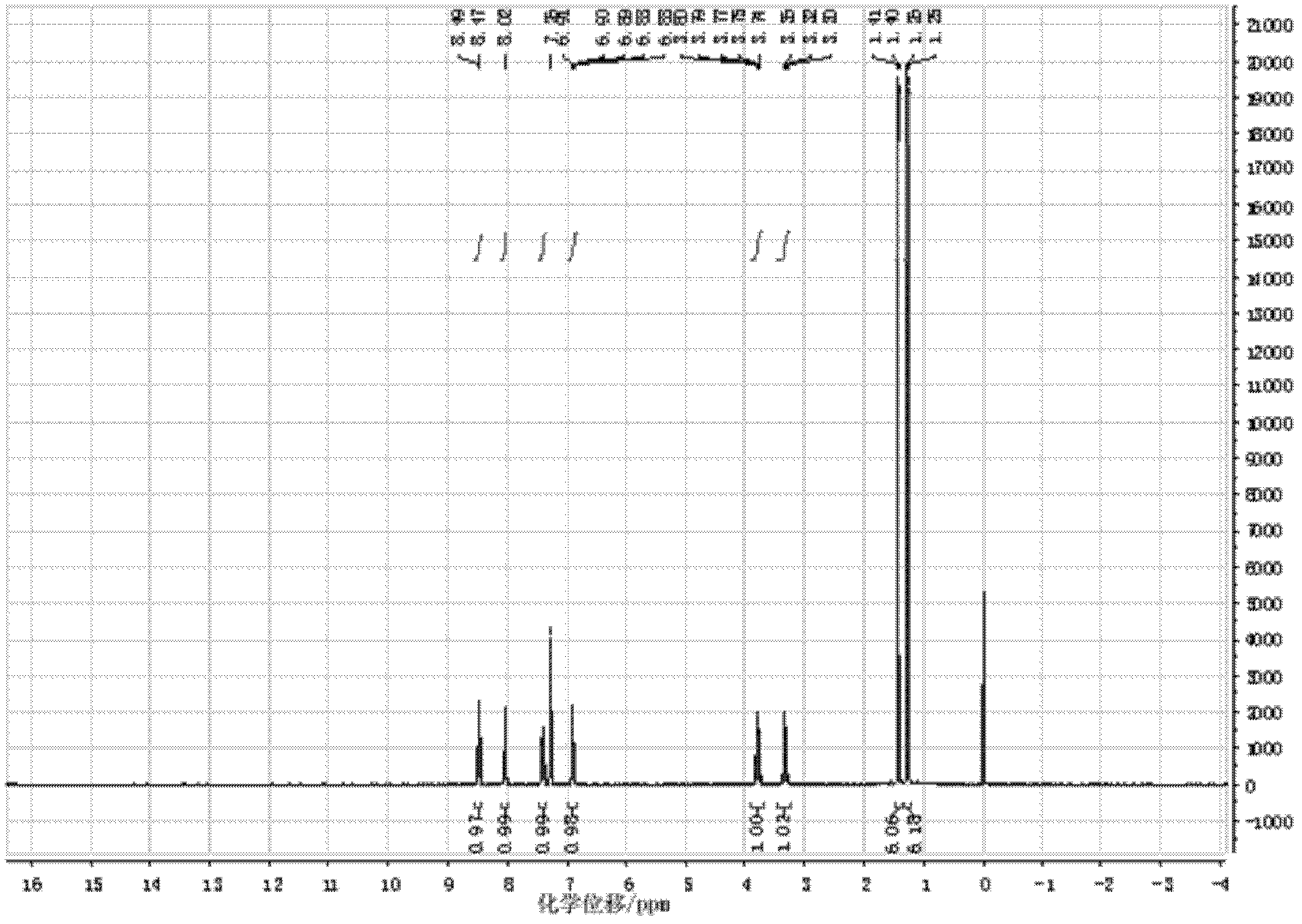
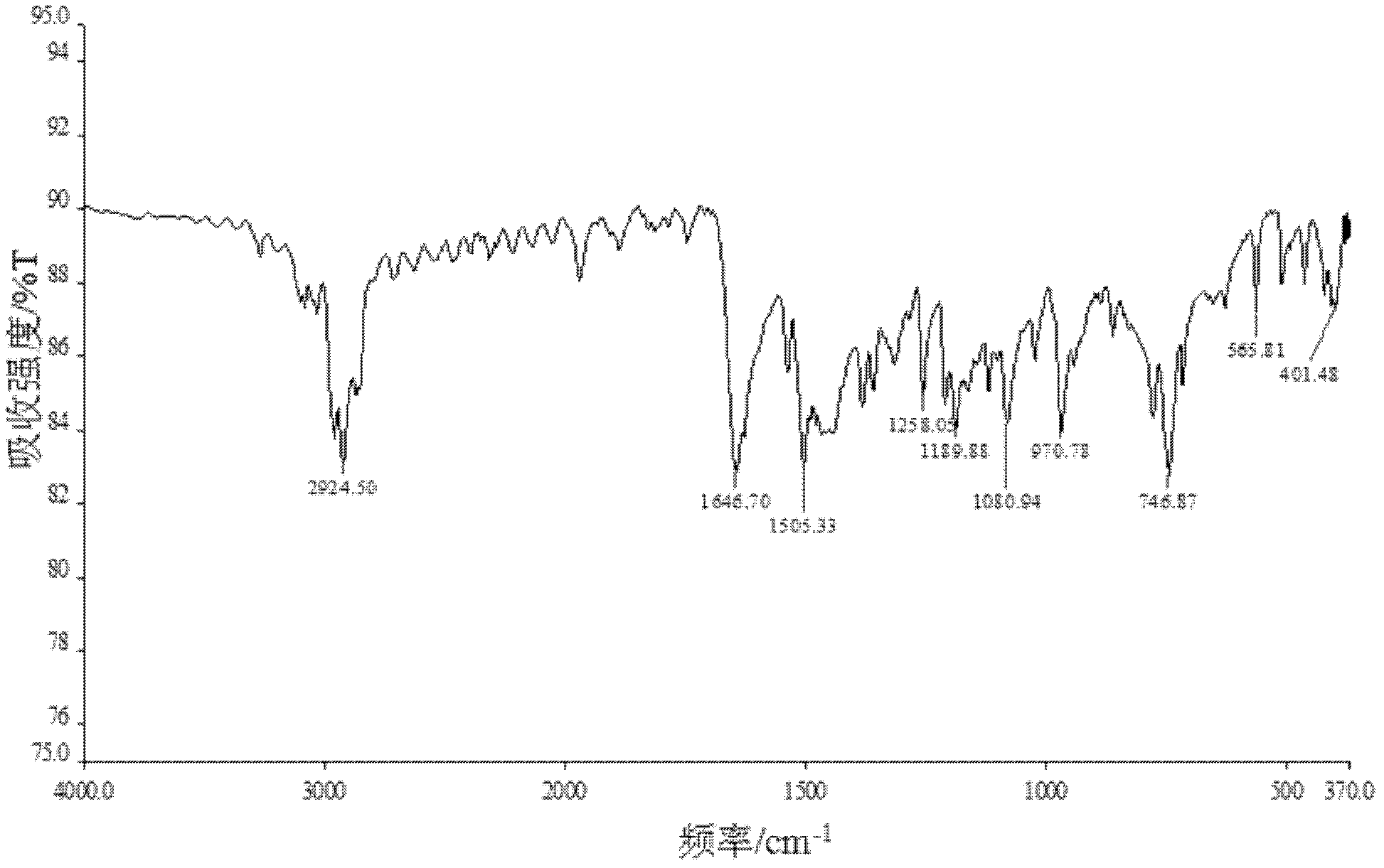
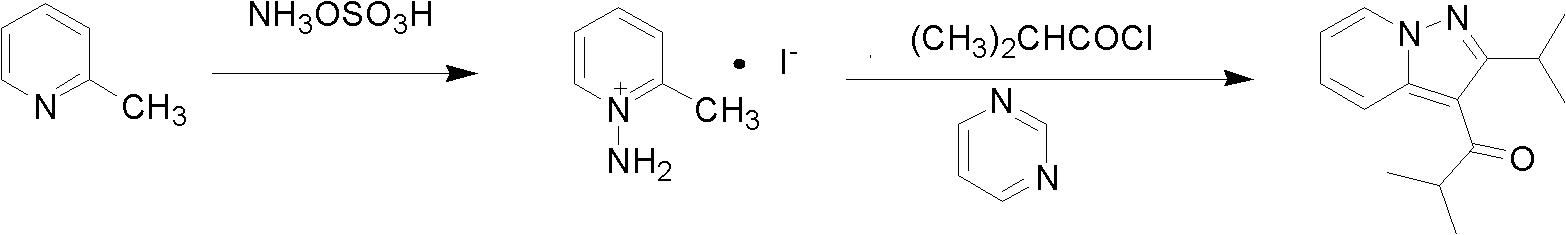
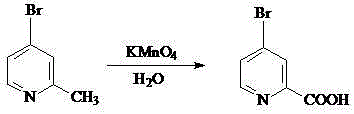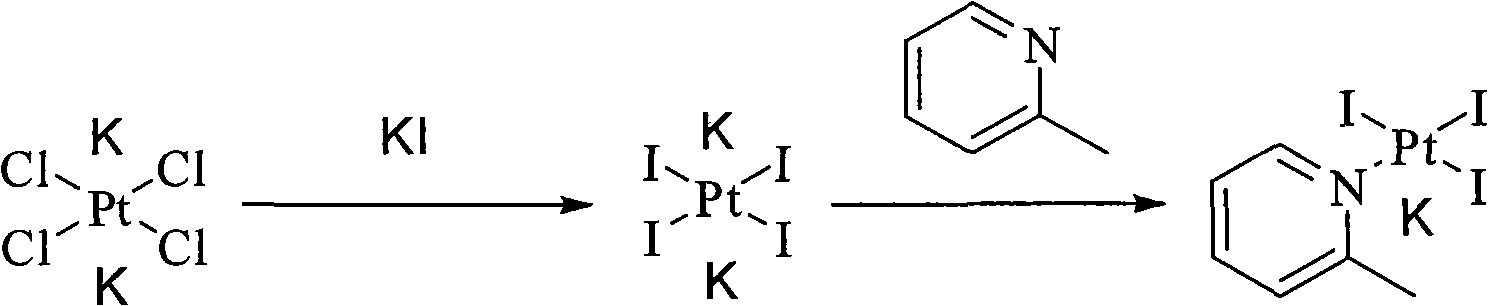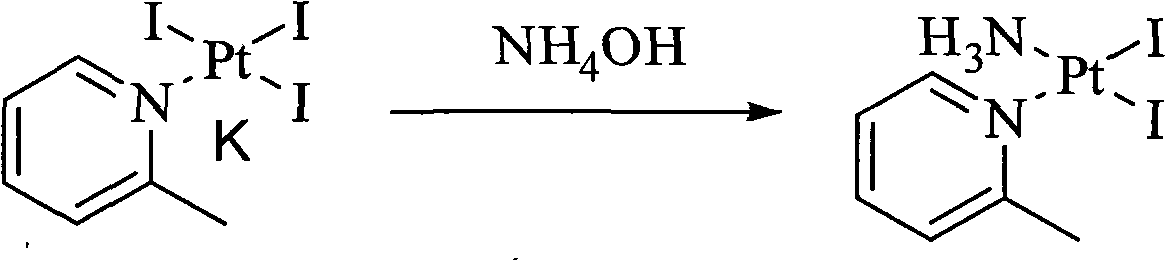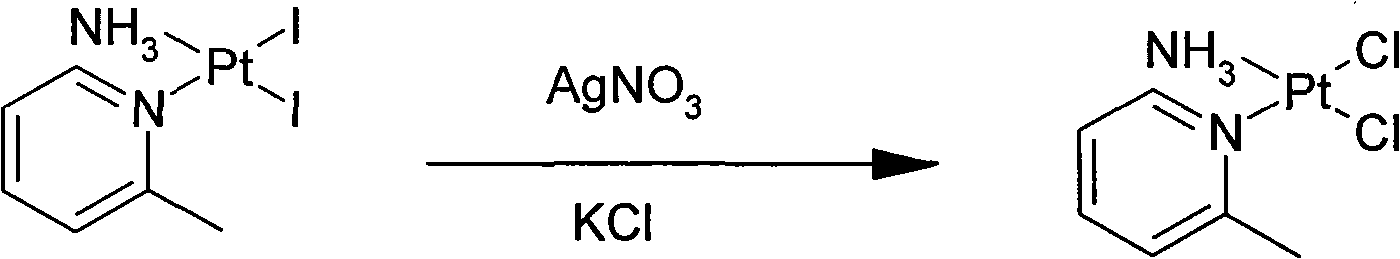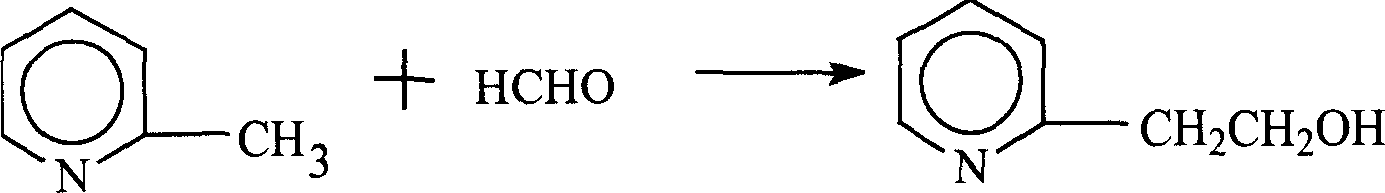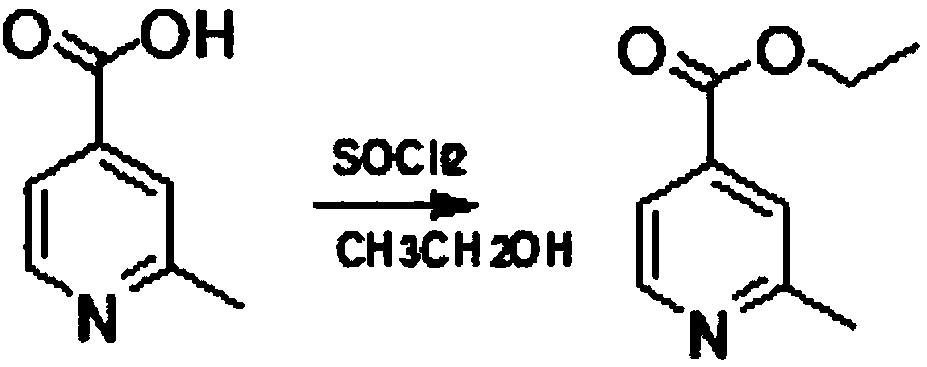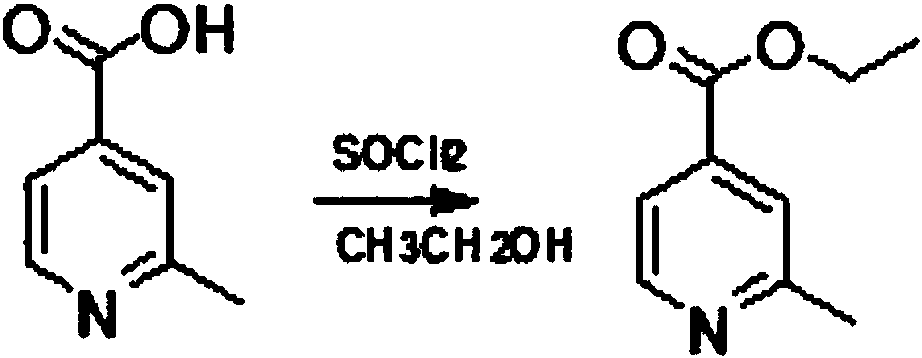Patents
Literature
34 results about "2-picoline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
2-Methylpyridine, or 2-picoline, is the compound described with formula C 6 H 7 N. 2-Picoline is a colorless liquid that has an unpleasant odor similar to pyridine.It is mainly used to make vinylpyridine and the agrichemical nitrapyrin.
Synthesis process of chronium 2-picolinate
The synthesis process of chromium 2-picolinate includes oxidizing 2-picoline into 2-picolinic acid, separating side product chromium oxide and mirabilite, and complexing with trivalent chromium salt to produce chromium 2-picolinate. The synthesis process of the present invention has simple operate, cheap material, high additional value of the side product, mild reaction condition, short reaction time, low power consumption, low cost, high yield and easy application in production.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
2-pyridinemethanol and synthetic method thereof
InactiveCN105153019ASimplify synthetic production stepsEmission reductionOrganic chemistryChemical recyclingAcetic acidAcetic anhydride
The invention discloses 2-pyridinemethanol and a synthetic method thereof. According to the method, 2-picoline and hydrogen peroxide are taken as raw materials, glacial acetic acid is taken as a solvent, the mixture has a reaction for 3-5 hours at the temperature of 70 DEG C-80 DEG C under the action of a catalyst, and 2-picoline nitrogen oxide is obtained after separation; 2-picoline nitrogen oxide and acetic anhydride react for 3-6 hours under the reflux condition, and acetic acid-2 pyridine methyl ester is obtained after separation; acetic acid-2 pyridine methyl ester is directly hydrolyzed under the alkali separating condition by sodium hydroxide, and a product 2-pyridinemethanol is obtained after separation. 2-pyridinemethanol and the synthetic method have the benefits as follows: the selectivity of process target products is high, material loss is low, the product content and the total yield can reach 98.5% and 65% respectively, and 2-pyridinemethanol is suitable for industrial mass production.
Owner:ANHUI COSTAR BIOCHEM CO LTD
Synthesis method of 2-picoline
InactiveCN105218431AImprove the industrial chainImprove survivabilityOrganic chemistryHeterogenous catalyst chemical elementsAlcoholSynthesis methods
The invention discloses a synthesis method of 2-picoline. According to the synthesis method, pyridine and methyl alcohol serve as raw materials, and Fe-MnOx-Yb serves as a catalyst. The synthesis method has the advantages that 2-picoline is prepared through one-step synthesis with pyridine and methyl alcohol as the raw materials, a pyridine industry chain is fully completed, the market dependency of pyridine bases of enterprises is reduced, controllable self-adjustability is achieved, and the survival ability of the enterprises is improved; the catalytic activity of the catalyst doped with Yb metal elements is improved by 3-5 times under influences of the characteristics (structure and electron cloud) of the Yb metal elements, the reaction period is shortened, and efficiency is improved; the synthesis method is efficient, the reaction ingredients are simple and easy to treat, energy is saved, and cost is lowered. The synthesis method is an environment-friendly and efficient synthesis technology, the product yield reaches up to 88%, and the pyridine conversion rate reaches 95%.
Owner:ANHUI COSTAR BIOCHEM CO LTD
Method for preparing pyridine bases
InactiveCN102020603AReduce consumptionEasy to operateOrganic chemistryChemical industryChemical synthesisFixed bed
The invention relates to a method for preparing pyridine bases. The pyridine bases are prepared by taking formaldehyde, acetaldehyde and ammonia as raw materials through the steps of carrying out chemical synthesis on the formaldehyde, the acetaldehyde and the ammonia in a fixed bed reactor in the presence of a catalyst at a temperature of 350 to 550 DEG C so as to obtain a high-temperature gas containing the pyridine bases; condensing the high-temperature gas so as to obtain a solution containing the pyridine bases, and carrying out ammonia absorption and steam stripping on the uncondensable ammonia-containing gas; then recovering the obtained ammonia and feeding the ammonia to the fixed bed reactor to be used as raw materials; extracting the obtained solution by benzene, then respectively obtaining a benzene solution containing the pyridine bases and a water solution containing a small amount of ammonia, benzene and pyridine bases, etc., after the solutions are processed, feeding the solutions to a combustion furnace to burn at a temperature of 1000 to 1200 DEG C; then discharging the solutions; feeding the benzene solution containing the pyridine bases into a benzene stripper to carry out steam stripping, then respectively obtaining benzene and pyridine base liquor, feeding the pyridine base liquor into different rectifying towers to carry out rectification under normal pressure or negative pressure, then respectively obtaining pyridine, 3-picoline and 2-picoline. The method in the invention has the advantages of low material consumption, high total yield, less catalyst consumption, and low energy consumption, and is simple in operation.
Owner:郑廷来
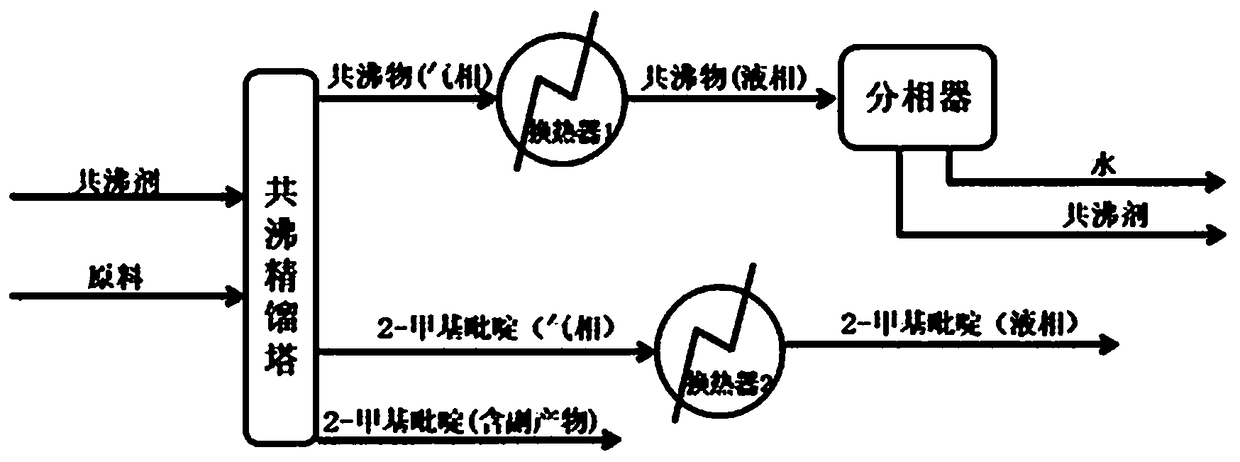
Method for realizing 2-picoline dehydration by side withdrawal from azeotropic distillation column
ActiveCN108191743AEasy to operateReduce cost inputOrganic chemistryChemical industryDistillation methodAzeotropic distillation
The invention provides a method for realizing 2-picoline dehydration by side withdrawal from an azeotropic distillation column. According to the method, cyclohexane is taken as an entrainer, a new lowazeotrope is formed from cyclohexane and water and breaks azeotropy of 2-picoline and water. A cyclohexane and water gas-phase mixture is obtained from top of the azeotropic distillation column, vapor rich in 2-picoline is withdrawn from the side of a column plate close to the column bottom and condensed into a liquid by a heat exchanger, a small amount of 2-picoline containing impurities is withdrawn from the column bottom, and accordingly, separation of 2-picoline and water is realized with the distillation method. The method is applicable to continuous distillation operation, 2-picoline with higher purity can be obtained by side withdrawal, 2-picoline is prevented from being heated at the column bottom for too long time to produce side effects, only one substance, namely, the entraineris introduced in the whole process, the entrainer can be efficiently recycled, and the method is a separation method with high efficiency and low energy consumption and cost.
Owner:淄博高新技术产业开发区精细化工和高分子材料研究院 +1
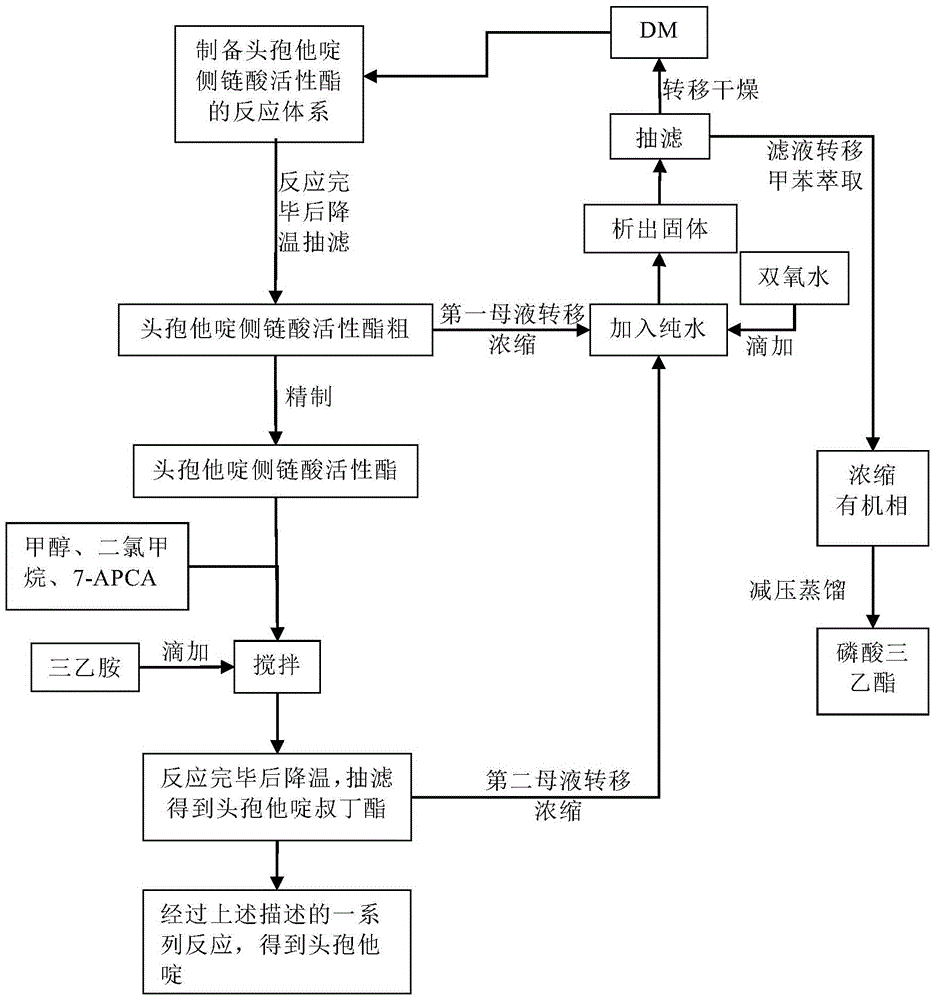
Original development quality ceftazidime and medicine preparation thereof
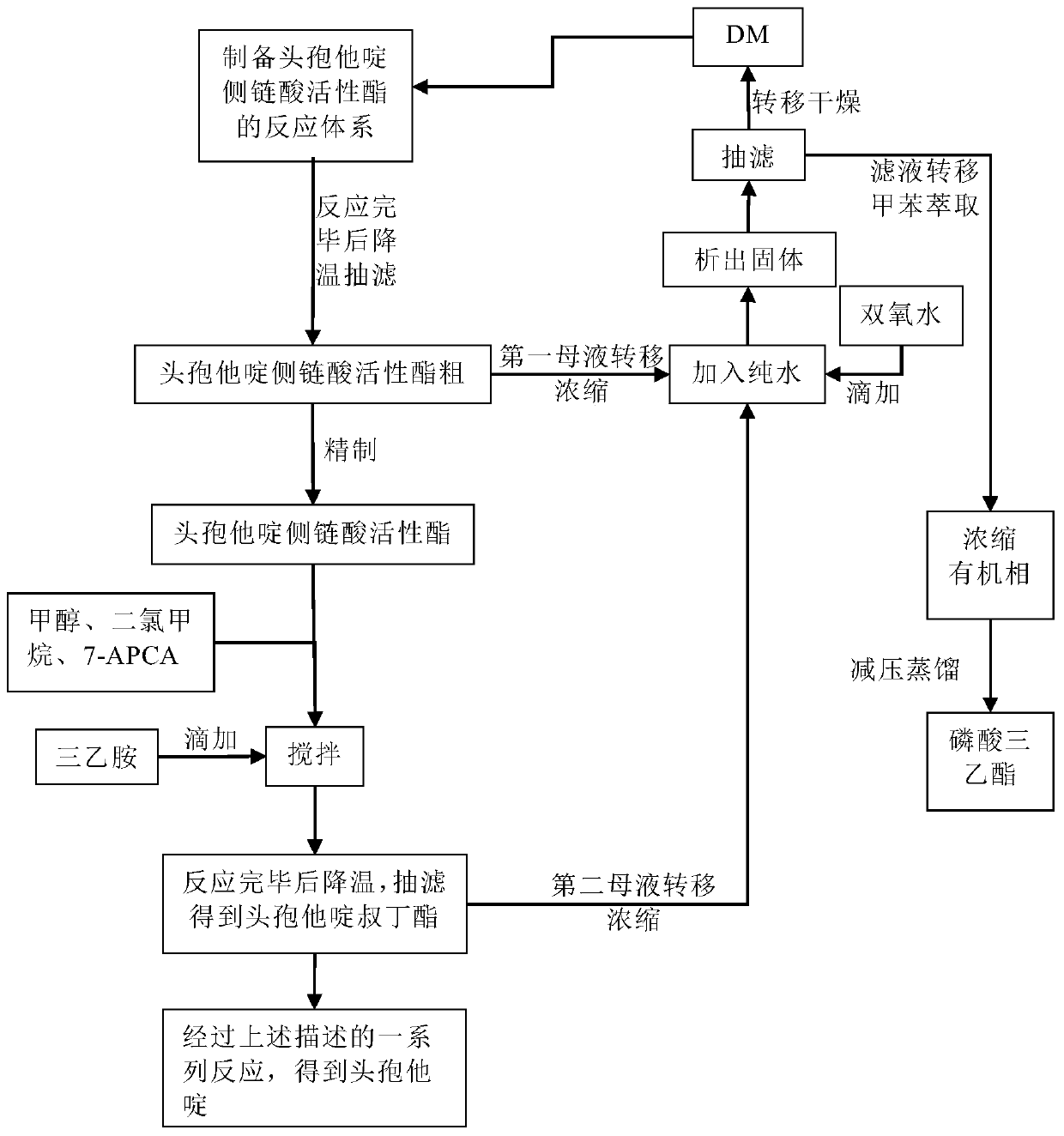
The invention discloses original development quality ceftazidime and a medicine preparation thereof. The third-generation cephalosporin antibiotics active ester midbody key technology and industrialization obtains the second prize of National Scientific and Technological Progress Award. The cephalosporin antibiotics active ester belongs to a key factor for influencing the internal quality of the cephalosporin. A preparation method comprises the following steps that (a) mixed solvents are added into ceftazidime side chain acid, dibenzothiazyl disulfide, aniline and 2-picoline; triethyl phosphate is dripped for reaction; (b) a coarse product is refined to obtain ceftazidime side chain acid active ester, and the first mother liquid is recovered; (c) the material is added into a mixed solvent for neutralizing 7-APCA; triethylamine is dripped; the temperature reduction is performed for crystal separation and filtering to obtain ceftazidime tert-butyl ester; the second mother liquid is recovered; (d) the ceftazidime tert-butyl ester is subjected to hydrolysis and purification, and then, the ceftazidime is obtained. The original development quality ceftazidime has the advantages that high-toxicity triphenylphosphine is not used; waste liquid and waste slag can be sufficiently recovered and reutilized; the method is safe; the cost is low; the yield is high; the industrial production is facilitated.
Owner:广东金城金素制药有限公司 +1
Catalyst for preparing 2-cyanopyridine by ammoxidation
ActiveCN107537537AHigh selectivityReduce generationOrganic chemistryCatalyst activation/preparationOrganic chemistryAmmoxidation
The invention relates to a catalyst for preparing 2-cyanopyridine by ammoxidation. The catalyst is prepared by taking TiO2 as a carrier and loading MoO3 and P2O5 by an impregnation method. The catalyst comprises 2.8-8.3% of MoO3 and 2.8-12.2% of P2O5 by mass percentage. The invention further discloses 2-cyanopyridine prepared by using the catalyst to catalyze the ammoxidation of 2-picoline. A testproves that a conversion rate of 2-picoline can reach above 98%, and the selectivity of 2-cyanopyridine can reach above 90% by the action of the catalyst.
Owner:HIGH & NEW TECH RES CENT OF HENAN ACAD OF SCI
Eco-friendly process for the preparation of 2-Chlorobenzylidene-Malononitrile (CS)
ActiveUS7732631B2Reduce sewage loadSpeed up the processCarboxylic acid nitrile preparationOrganic compound preparation4-MethylpyridineMorpholine
An improved process for the preparation of 2-chlorobenzylidenemalononitrile (CS) comprising of the steps of: preparing malononitrile suspension by adding 5-20% (wt %) preferably 12-14% malononitrile to water while constantly stirring and then adding 0.05-0.5% (v / v) preferably 0.1-0% of a catalyst like piperidine, pyridine, 2-picoline, 3-picoline, 4-picoline or morpholine preferably piperidine piperidine with constant stirring at 20-30° C.; condensing the malononitrile suspension prepared in step (a) with 2-chlorobenzaldehyde by adding 10-15% (w / v) preferably 25-30%, of 2-chlorobenzaldehyde cover a period at 30-45 minutes so that the temperature of the reaction mixture remains below 50° C., constantly stirring for 20-40 minutes, then filtering the CS and drying it at 20-30° C. under water vacuum for 3-5 hrs.
Owner:DIRECTOR GENERAL DEFENCE RES & DEV ORG
Preparation method of pyridine-2-formaldehyde
The invention discloses a preparation method of pyridine-2-formaldehyde. The preparation method comprises the following steps of: preparing a catalyst containing an acidity regulator and a transition metal oxide carried on a carrier; carrying out a gas-phase oxidizing reaction in a fixed bed catalytic reactor at 250-400 DEG C to generate a crude target product by using 2-picoline, oxygen and water as raw materials; extracting the crude product through dichloromethane and then decompressing and distilling extract liquor to remove the dichloromethane; and then rectifying to obtain a pure product with the pyridine-2-formaldehyde content higher than 98.5 percent. The method has simple preparation process, low raw material cost, high catalytic oxidation reaction selectivity, easy separation of main products and byproducts and high purity.
Owner:ZHEJIANG UNIV
Method for manufacturing catalyst used for synthesizing 2-picoline
ActiveCN101108366AAchieve synthesisRealize storage and transportationOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsFuranVacuum pressure
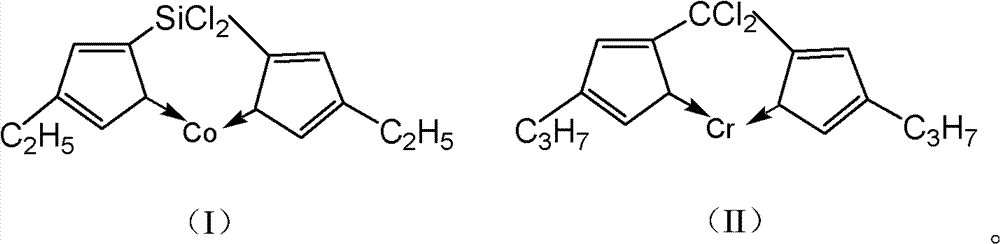
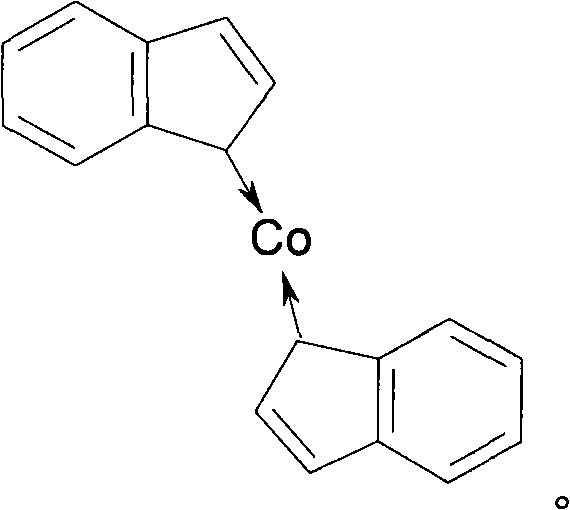
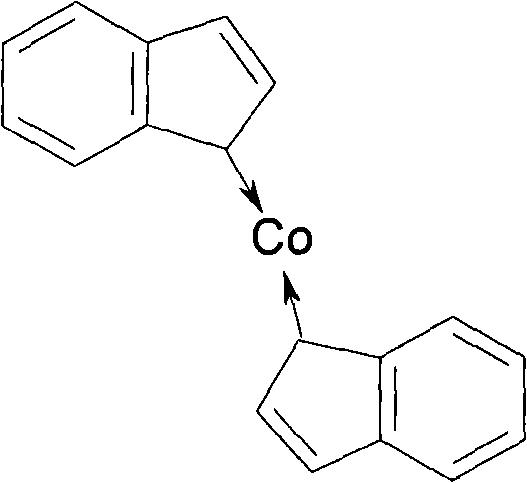
The invention discloses a catalyst with high safety used for synthesizing 2-picoline and the preparation method, the procedures are: add THF and NaNH2 in sodium salt kettle, drip CPD when the temperature is 0 to 45 DEG C. and keep the reaction for 0.5 to 3 hours to produce CPD sodium; add anhydrous CoCl2 in the synthesis kettle after adding tetrahydrophthalic anhydride furan, stir for 30 to 40 minutes then add the prepared CPD sodium, keep the backflow of the liquid at the temperature of 60 to 70 DEG C. and keep stirring reaction for 1 to 2 hours continuously, the reaction of preparing catalyst is finished; after the backflow ending, recycle solvent and increase the temperature to 65 to 75 DEG C., when the liquid flow volume is reduced, open vacuum pressure distill recycle solvent, stir and add acetonitrile continuously when having no liquid in the kettle, after stirring for 1 hours under the room temperature to remove sodium chloride and get the catalyst in mixture state. The invention has simple reaction process, which prevents the strict requirements and complication of the synthesis technique of organic Co catalyst crystal.
Owner:HEBI SAIKE CHEM ENG CO LTD
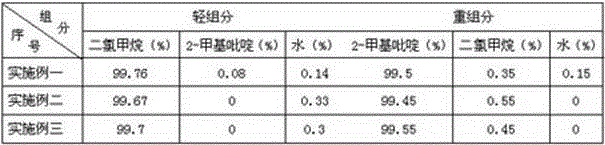
Method for realizing dehydration of 2-picoline through coupling of extraction and rectification
InactiveCN106748978AThe extraction operation consumes less energyReduce cost inputOrganic chemistryCoupling2-picoline
The invention discloses a method for realizing dehydration of 2-picoline through coupling of extraction and rectification, and belongs to the technical field of refinement of organic high molecular compounds. The method is characterized by comprising the following steps: (1) performing mixing: mixing dichloromethane with a to-be-dehydrated 2-picoline solution so as to obtain mixed liquor, wherein the mass ratio of the dichloromethane to the 2-picoline in the mixed liquor is (6-10) to 1; (2) performing extraction and phase separation: placing the mixed liquor in a phase separator for phase separation so as to obtain an aqueous phase and an organic phase and complete extraction once; (3) performing extraction and phase separation once again: detecting the aqueous phase obtained in the step (2), if the 2-picoline is greater than or equal to 1% by mass of the aqueous phase, adding the dichloromethane to the aqueous phase, and when the mass ratio of the dichloromethane to the 2-picoline is (6-10) to 1, executing the step (2) and the step (3); and (4) performing rectification: performing rectification on the organic phase obtained in the step (2) and the step (3), extracting an extraction agent at upper parts, and extracting 2-picoline at lower parts. The dehydration method has the advantages of good separation effects, low production cost, low energy consumption and the like.
Owner:山东海昆化工技术有限公司
Preparation method of benzimidazole proton pump inhibitor intermediate
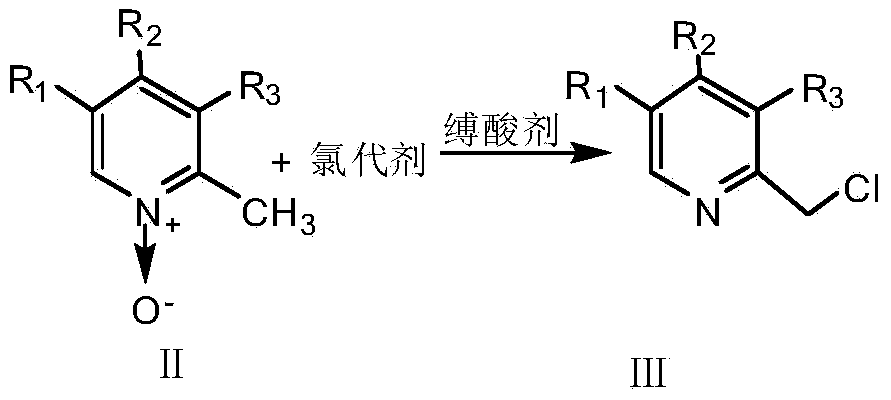
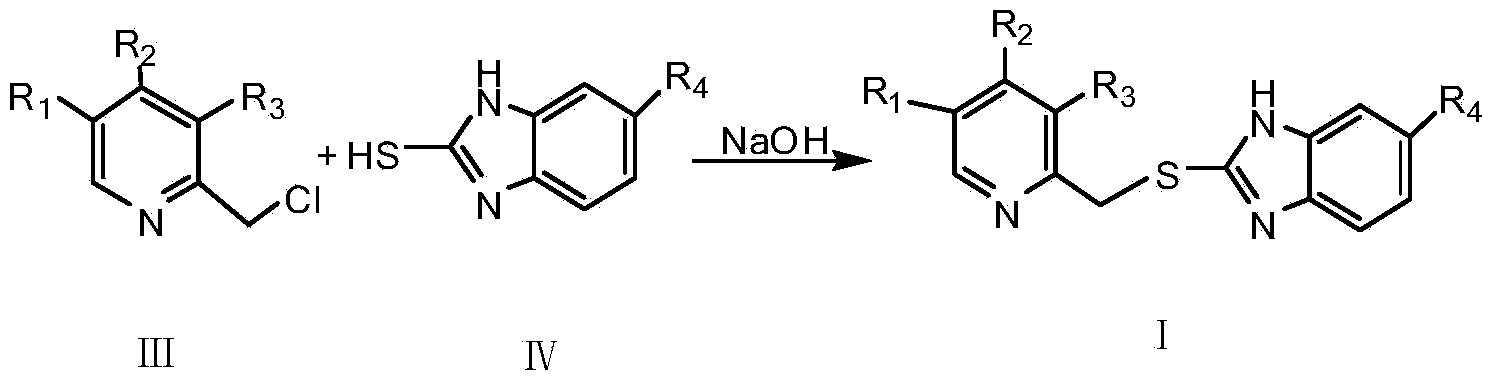
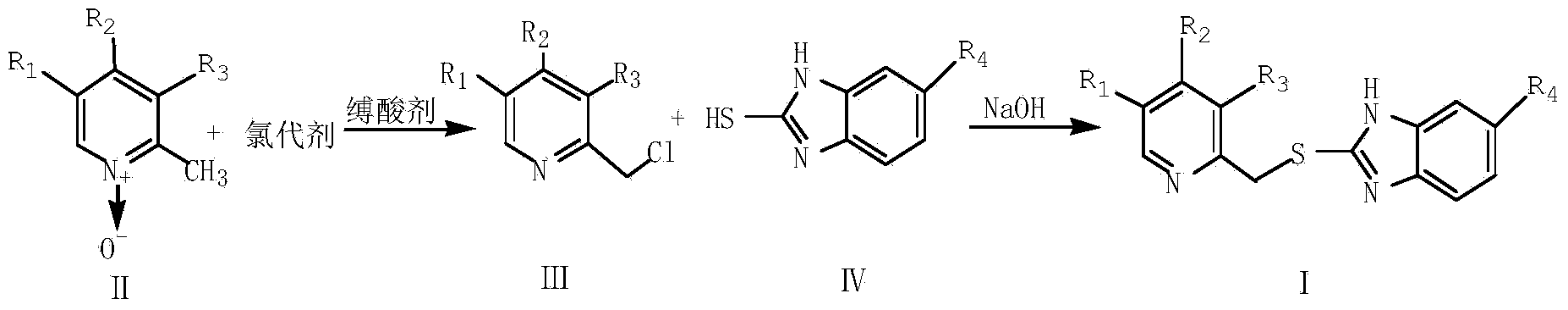
InactiveCN103664885ASimple and fast operationHigh reaction yieldOrganic chemistryBenzimidazole derivativeOrganic layer
The invention provides a preparation method of a benzimidazole proton pump inhibitor intermediate. The method comprises the following steps: (1)dissolving 2-picoline oxynitride derivative into an organic solvent, stirring, using dichloromethane to perform metered volume on a chlorinated agent and an acid-binding agent, dropwise adding the chlorinated agent solution firstly, then simultaneously dropwise adding the acid-binding agent solution, reacting, adjusting pH, filtering, extracting, drying and distilling to obtain 2-chlorine picoline derivative; (2)dissolving 2-sulfydryl-1-H-benzimidazole derivative into a sodium hydroxide ethanol solution, adding 2-chlorine picoline derivative, stirring for reaction, filtering, extracting by using dichloromethane, drying, distilling the organic layer, adding ethyl acetate, then adding petroleum ether, stirring, freezing crystallization, filtering, washing and drying to obtain the benzimidazole proton pump inhibitor intermediate. The invention has the following advantages: (1) the chlorinated performance of phosphorus oxychloride is excellent, other byproducts are not generated; (2) the reaction conversion rate is improved; (3) the reaction steps are simplified, and the product loss is reduced.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
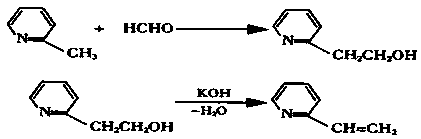
Method for preparing 2-vinylpyridine
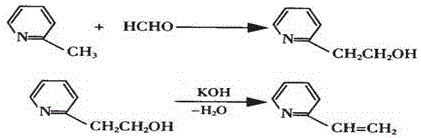
The invention provides a method for preparing 2-vinylpyridine. The 2-vinylpyridine is mainly prepared from 2-picoline and formaldehyde. The process for synthesizing the 2-vinylpyridine comprises 'two-step' and 'one-step' production processes, and comprises the steps of condensing 2-picoline and formaldehyde to produce hydroxyethyl pyritine; and dehydrating the hydroxyethyl pyritine at a certain temperature in the action of a catalyst to generate the 2-vinylpyridine. The 'one-step' production process is adopted, 2-vinylpyridine is further generated at a certain temperature under the action of an acid catalyst by adopting 2-picoline and formaldehyde as raw materials, the process flow is simple, the yield is high, and the consumption of raw materials, auxiliary materials and energy of the 'one-step' method is superior to those of the 'two-step' method.
Owner:JIANGSU YATAI CHEM
Xylose staphylocci for degrading 2-picoline and its use
The Staphylococcus xylous B1 is prepared by separating out from active sludge in sewage disposal tank of petrochemical plant, purifying and culturing. The preservative number is CGMCC No. 1485. It has excellent efficient degradable performance for 2-picoline and can be used to dispose waste water and waste gas containing 2-picoline, polluted water and soil.
Owner:CHINA PETROLEUM & CHEM CORP +1
Eco-Friendly Process for the Preparation of 2-Chlorobenzylidene-Malononitrile (Cs)
ActiveUS20080139837A1Reduce sewage loadSpeed up the processCarboxylic acid nitrile preparationOrganic compound preparation4-MethylpyridineMorpholine
An improved process for the preparation of 2-chlorobenzylidenemalononitrile (CS) comprising of the steps of: preparing malononitrile suspension by adding 5-20% (wt %) preferably 12-14% malononitrile to water while constantly stirring and then adding 0.05-0.5% (v / v) preferably 0.1-0% of a catalyst like piperidine, pyridine, 2-picoline, 3-picoline, 4-picoline or morpholine preferably piperidine piperidine with constant stirring at 20-30° C.; condensing the malononitrile suspension prepared in step (a) with 2-chlorobenzaldehyde by adding 10-15% (w / v) preferably 25-30%, of 2-chlorobenzaldehyde cover a period at 30-45 minutes so that the temperature of the reaction mixture remains below 50° C., constantly stirring for 20-40 minutes, then filtering the CS and drying it at 20-30° C. under water vacuum for 3-5 hrs.
Owner:DIRECTOR GENERAL DEFENCE RES & DEV ORG
Mixed metal complex constructed by tris(2-picoline) amine and preparing method thereof
The invention relates to a synthetic method for a terbium and molybdenum mixed metal complex containing tris(2-picoline) amine. The chemical composition of the complex contains tpma ligand, wherein tpma is equal to tris(2-picoline) amine. According to the synthesis scheme, ligand tris(2-picoline) amine and TbCl3.6H2O are prepared into methanol solutions respectively according to the stoichiometric ratio, the methanol solutions are mixed and filtered after being stirred for 2 h at the normal temperature, a beaker containing the filter liquor is placed into a container containing isopropyl ether, a gas-liquid interface diffusion method is adopted for obtaining a Tb(tpma)Cl3 complex, the Tb(tpma)Cl3 complex and K3[Mo(CN)8] are prepared into a water solution according to the stoichiometric ratio, the mixed liquor is stirred for 2 h at the normal temperature after being mixed, filtering is carried out, the filter liquor stands in air, and volatilizes slowly, and a target product can be obtained after about two weeks.
Owner:TIANJIN POLYTECHNIC UNIV
Preparation method for 5-bromine-2-picolinic acid
InactiveCN104987308ALow priceMild reaction conditionsOrganic chemistryOrganic synthesisReaction temperature
The invention belongs to the field of organic synthesis, and particularly relates to a preparation method for 5-bromine-2-picolinic acid. The preparation method for the 5-bromine-2-picolinic acid comprises the following steps that 5-bromine-2-picoline is used as raw material, water is used as solvent, the temperature is raised to 80 DEG C by heating, potassium permanganate is added in batches, the reaction temperature is maintained to be 85 to 90 DEG C, the heating time is 60 to 100 min, and distilling, filtering, pH value adjusting and cooling crystallization are conducted on reactant. The preparation method for the 5-bromine-2-picolinic acid has the advantages that the reaction condition is mild, the operation is easy, aftertreatment is simple, large-scale production is easy, and the preparation method for the 5-bromine-2-picoline is especially suitable for industrial production; the yield rate is high, the price of raw materials is low, and the production cost is low.
Owner:林达钦
Method for extracting 2-picoline and 3-picoline from coking crude benzene
The invention discloses a method for extracting 2-picoline and 3-picoline from coking crude benzene. According to the method, the 2-picoline and the 3-picoline are extracted by taking the coking crude benzene as a raw material, and simultaneously, benzene, methylbenzene and dimethylbenzene are obtained, the utilization rate of the raw material coking crude benzene is increased, and the yield is increased; the technological process of the method is advanced; desalted water can be repeatedly recycled in the whole production process, no waste is generated, environmental problem is avoided, and the method is environment-friendly; and the material has little corrosion on equipment and is low in cost and high in revenue efficiency.
Owner:WEIFANG YUANLI CHEM
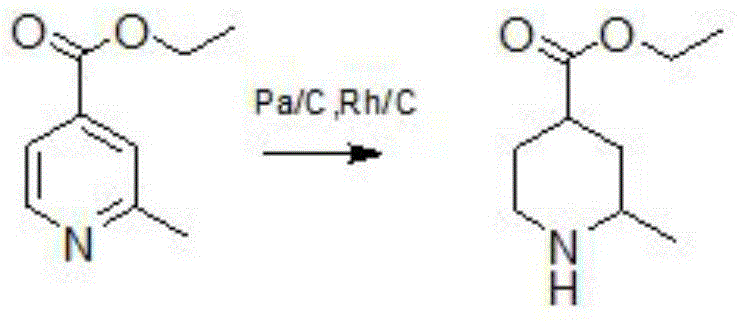
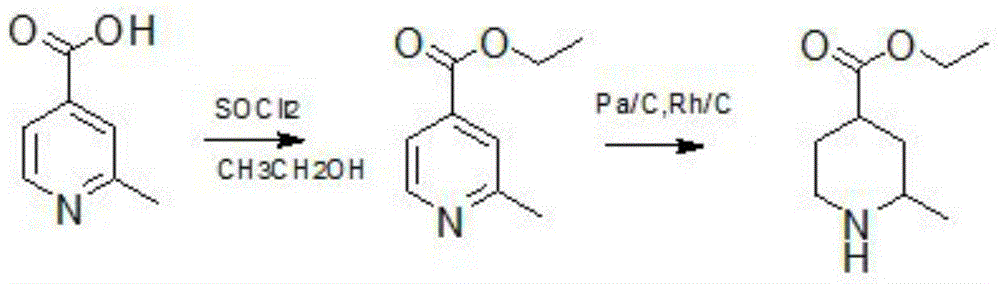
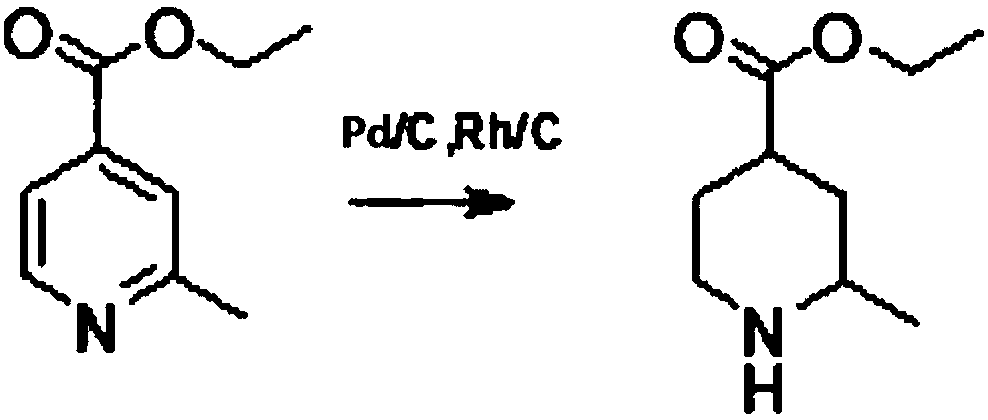
Method for reducing pyridine ring to piperidine in 2-picoline-4-formic acid
The invention discloses a method for reducing a pyridine ring to piperidine in 2-picoline-4-formic acid. The method comprises the step one of preparation of 2-picoline-4-ethyl formate, wherein the initiator 2-picoline-4-formic acid is dissolved into ethyl alcohol solvent, thionyl chloride is dropped into the mixture to carry out esterification reaction to obtain esterification product mixing liquid, and after-treatment is carried out on the esterification product mixing liquid to obtain the 2-picoline-4-ethyl formate; the step two of reduction reaction, wherein the 2-picoline-4-ethyl formate generated through the reaction in the former step is pressurized under the effect of a catalyst, hydrogen is introduced into the 2-picoline-4-ethyl formate to carry out reduction reaction to obtain a reduction product mixing liquid, the addition quantity of the catalyst is 10%-20% of the content of the initiator, and the catalyst is a mixture of palladium carbon and rhodium carbon; the step three of after-treatment, wherein the reaction product mixing liquid reacted in the step two is treated to obtain a 2-picoline-4-ethyl formate product. The new method for producing the 2-picoline-4-ethyl formate has the advantages of being high in efficiency, high in reaction speed and simple in process.
Owner:WUHAN KONBERD BIOTECH
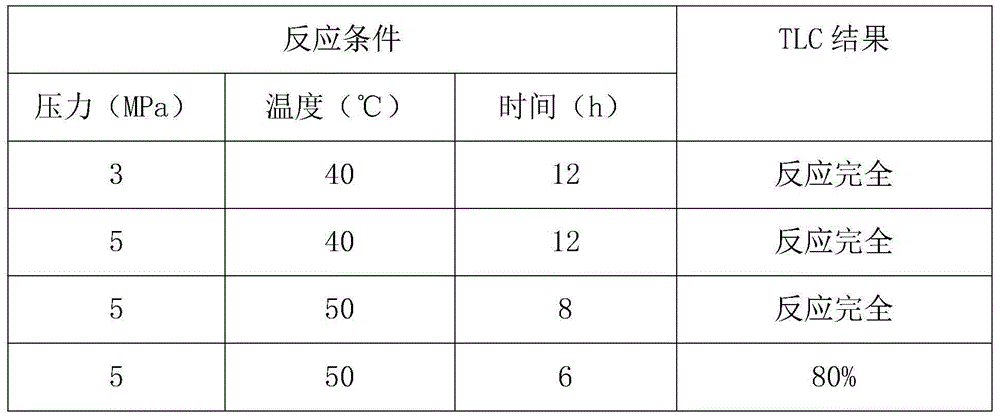
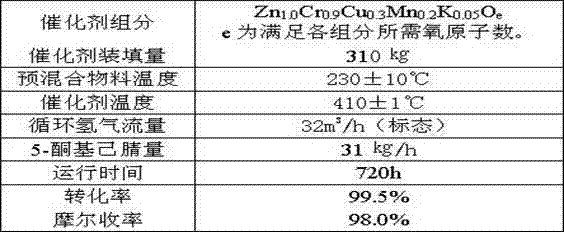
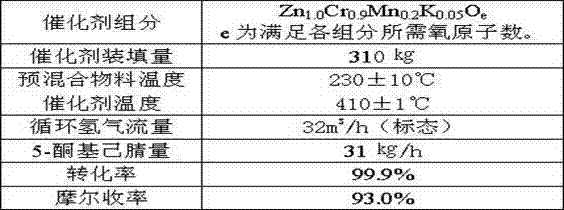
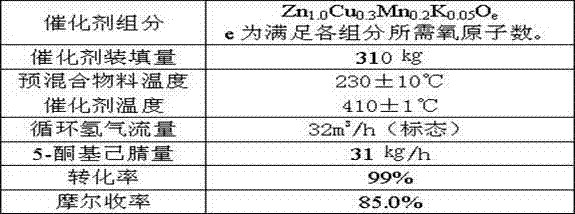
Method of synthesis of 2-picoline through 5-ketohexanenitrile
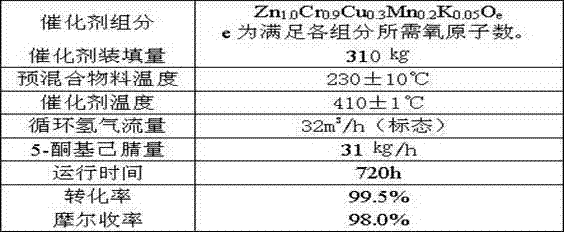
ActiveCN102924368AReasonable designEasy to operateOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsHydrogenDehydrogenation
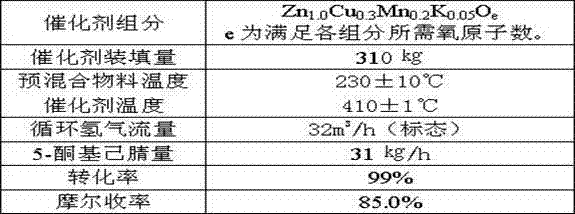
The invention provides a method of synthesis of 2-picoline through 5-ketohexanenitrile. According to the method, 5-ketohexanenitrile (acetyl butyronitrile) is utilized as raw materials, with the existence of hydrogen, a particle catalyst is filled in a fixed bed, at the temperature of 380-420 DEG C, under the pressure of 0.02-0.1Mpa, the 2-picoline is synthesized in one step, and each active component of the particle catalyst is Zn1.0CraCubMncKdOe in terms of atomicity. According to the method, with the existence of hydrogen, the 5-ketohexanenitrile raw materials are subjected to hydrogenation, ring closure and dehydrogenation to generate the 2-picoline meanwhile. The method is reasonable in design, high in operability and safe to operate. Due to the method, waster water, waste and waste solid are not generated, and requirement of environment-friendly chemical industry at present is met. According to the method, composite zinc-chromium-manganese-potassium is used as a catalyst, conversion ratio of the 5-ketohexanenitrile can reach 99.0%, molar yield of the 2-picoline can reach 99.0%, and the method can be directly used for synthesizing 2-cyanopridine.
Owner:连云港阳方催化科技有限公司
New method for preparing picoplatin
ActiveCN101775040ASolubility product is smallEasy to removeGroup 8/9/10/18 element organic compoundsSilver iodidePicoplatin
The invention relates to a novel method for preparing picoplatin. The technical scheme adopts following steps of: firstly generating potassium tetraiodoplatinate by reacting potassium tetrachloro platinum with potassium iodide to improve the activity of reactants; generating triiodo (2-picoline) platinum (II) potassium by reacting the potassium tetraiodoplatinate with 2- picoline; generating Cis-2 iodine-ammonia and (2-picoline) platinum (II) by reacting triiodo (2-picoline) platinum (II) potassium with ammonia water; adding the Cis-2 iodine-ammonia and the (2-picoline) platinum (II) together to deionized water; stirring the deionized water for reaction at the room temperature; filtering silver iodide precipitate when the reaction is completed; and adding potassium chloride so as to gradually separate out crystallized products of the picoplatin. In the invention, the reaction is more complete, the utilization of the platinum and the yield are enhanced, the products do not have silver irons, and the problem that the silver irons are exceeded needs not to be worried.
Owner:NANJING CHENGONG PHARM CO LTD
Triamine monomer containing pyridine structure and preparation method thereof
The invention discloses a triamine monomer containing a pyridine structure and a preparation method thereof and belongs to the field of polymer synthesis. 1,1,1-tris(4-hydroxyphenyl)ethane serves as raw materials to react with a nitro compound containing pyridine groups with a polar solvent as a solvent and alkaline as a catalyst, an aromatic nitro compound is synthesized, and an intermediate product obtained after a reaction is catalyzed with a catalyst and subjected to hydrazine hydrate reduction so that finally the triamine monomer can be obtained. The nitro compound can be 2-chloro-5-nitropyridine, 2-chloro-5-nitro-3-picoline, 2-chloro-5-nitro-4-picoline and 6-chloro-3-nitro-2-picoline, alkali can be potassium hydroxide, sodium hydroxide, potassium carbonate, sodium carbonate, potassium acetate, sodium acetate and sodium bicarbonate. The monomer has high reaction activity; the preparation technology is simple, the yield is high, and purity is high; a better effect is achieved in the aspect of large-molecular-weight hyperbranched polymer preparation, and broad application prospects are achieved.
Owner:JILIN UNIV
Transition metal catalyst and method for preparing picoline through adopting catalyst
ActiveCN101693211BHigh catalytic efficiencyGood production safetyOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCyanideAcetonitrile
The invention provides a transition metal catalyst, wherein the structural general formula of the catalyst is R1(Z)yMR2, wherein R1 and R2 are same or different, and are respectively substituted or unsubstituted cyclopentadienyl, substituted or unsubstituted indenyl and substituted or unsubstituted fluorenyl, wherein y is 0 or 1, Z is a linked group for connecting R1 and R2, and M is transition metal element. The invention further provides a method for preparing picoline, wherein the method comprises leading acetylene and methyl cyanide to contact for reacting under the existence of catalyst,and the catalyst is the transition metal catalyst supplied by the invention. The transition metal catalyst can effectively prompt methyl cyanide and acetylene to react for preparing 2-picoline.
Owner:北京颖泰嘉和分析技术有限公司
Load type transition metal catalyst and preparation method thereof and method for preparing picoline
ActiveCN101693210AEasy to prepareEasy to manufactureOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSolventCoordination complex
The invention provides a load type transition metal catalyst, which comprises a porous support and a transition metal complex which is loaded on the porous support, the general formula of the transition metal complex is R1(Z)yMR2, wherein R1 and R2 are the same or different, and are respectively substituted or unsubstituted cyclopentadienyl, substituted or unsubstituted indenyl and substituted or unsubstituted fluorenyl, wherein y is 0 or 1, Z is a linked group for connecting R1 and R2, and M is transition metal element. The invention further provides a method for preparing load type transition metal catalyst, which comprises leading the porous support and the transition metal complex to contact under the existence of dissolvent, and removing dissolvent. The invention further provides a method for preparing picoline through adopting load type transition metal catalyst. The load type transition metal catalyst can effectively prompt methyl cyanide and acetylene to react for preparing 2-picoline.
Owner:NUTRICHEM LAB CO LTD
Preparation method of ibudilast
InactiveCN102617579AReduce typesReduce the use effectOrganic chemistryRespiratory disorderDistillationSolvent
A preparation method of ibudilast relates to medicine compound compounding and aims at resolving the problem that the existing preparation method of the ibudilast is difficult to operate or low in yield and cannot achieve industrial production accordingly. The method includes 1 preparing 1-amino-2-picoline oxide and 2 adopting the 1-amino-2-picoline oxide to prepare the ibudilast. The method has the advantages that the preparation method is convenient in post-processing, the yield is 80% to 95%, and type and quantity of used solvent in industrialization are reduced; 2 medicine and agents used in the method are low in price, easy to obtain, high in yield and low in cost, thereby being suitable for industrialized production; and 3 a final product of the method is prepared by reduced pressure distillation and purification, and the purity can reach over 99%. The preparation method is mainly used in preparation of the ibudilast.
Owner:HEILONGJIANG UNIV
Method of synthesis of 2-picoline through 5-ketohexanenitrile
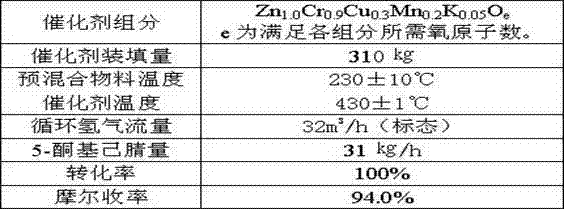
ActiveCN102924368BReasonable designEasy to operateOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsChemical industryPotassium
The invention provides a method of synthesis of 2-picoline through 5-ketohexanenitrile. According to the method, 5-ketohexanenitrile (acetyl butyronitrile) is utilized as raw materials, with the existence of hydrogen, a particle catalyst is filled in a fixed bed, at the temperature of 380-420 DEG C, under the pressure of 0.02-0.1Mpa, the 2-picoline is synthesized in one step, and each active component of the particle catalyst is Zn1.0CraCubMncKdOe in terms of atomicity. According to the method, with the existence of hydrogen, the 5-ketohexanenitrile raw materials are subjected to hydrogenation, ring closure and dehydrogenation to generate the 2-picoline meanwhile. The method is reasonable in design, high in operability and safe to operate. Due to the method, waster water, waste and waste solid are not generated, and requirement of environment-friendly chemical industry at present is met. According to the method, composite zinc-chromium-manganese-potassium is used as a catalyst, conversion ratio of the 5-ketohexanenitrile can reach 99.0%, molar yield of the 2-picoline can reach 99.0%, and the method can be directly used for synthesizing 2-cyanopridine.
Owner:连云港阳方催化科技有限公司
Process for large-scale preparation of 2-hydroxyethyl pyridine
The invention relates to a process for preparing 2-hydroxyethylpyridine on an industrial scale. 2-methylpyridine is used as the starting material; in the presence of catalyst benzoic acid, chloroacetic acid or acetic acid, it is condensed with paraformaldehyde to generate 2-hydroxyethylpyridine; the weight ratio of raw materials is: 2-methylpyridine : Paraformaldehyde: Catalyst=1: 0.03~0.12: 0.0024~0.012; Reaction condition: 90~180 ℃ / 10~30h under agitation: Reaction is finished, vacuum distillation, collection boiling range 60~150 ℃ / 10~100mmHg distillate Parts, to obtain 2-hydroxyethylpyridine finished product. It fills the gap in the country, and the production process is simple; the yield is high, and the yield can reach 84-94.85% based on 2-picoline.
Owner:淄博张店东方化学股份有限公司
A kind of original research quality ceftazidime and pharmaceutical preparation thereof
ActiveCN105646541BHigh purityHigh yieldAntibacterial agentsOrganic active ingredientsTriethylphosphiteSlag
The invention discloses original development quality ceftazidime and a medicine preparation thereof. The third-generation cephalosporin antibiotics active ester midbody key technology and industrialization obtains the second prize of National Scientific and Technological Progress Award. The cephalosporin antibiotics active ester belongs to a key factor for influencing the internal quality of the cephalosporin. A preparation method comprises the following steps that (a) mixed solvents are added into ceftazidime side chain acid, dibenzothiazyl disulfide, aniline and 2-picoline; triethyl phosphate is dripped for reaction; (b) a coarse product is refined to obtain ceftazidime side chain acid active ester, and the first mother liquid is recovered; (c) the material is added into a mixed solvent for neutralizing 7-APCA; triethylamine is dripped; the temperature reduction is performed for crystal separation and filtering to obtain ceftazidime tert-butyl ester; the second mother liquid is recovered; (d) the ceftazidime tert-butyl ester is subjected to hydrolysis and purification, and then, the ceftazidime is obtained. The original development quality ceftazidime has the advantages that high-toxicity triphenylphosphine is not used; waste liquid and waste slag can be sufficiently recovered and reutilized; the method is safe; the cost is low; the yield is high; the industrial production is facilitated.
Owner:广东金城金素制药有限公司 +1
Method for reducing pyridine ring to piperidine in 2-methylpyridine-4-carboxylic acid
Owner:WUHAN KONBERD BIOTECH
Method for manufacturing catalyst used for synthesizing 2-picoline
ActiveCN101108366BAchieve synthesisRealize storage and transportationOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsDistillationSodium salt
The invention discloses a catalyst with high safety for synthesizing 2-methyl pyridine and a preparation method for the catalyst. The preparation method comprises the following steps: tetrahydrofuran and sodamide are added in a sodium salt kettle, cyclopentadiene is dripped at temperature of 0 to 45 DEG C, the reaction is kept for 0.5 to 3 hours to prepare cyclopentadienylsodium; anhydrous cobaltous chloride is added in the synthesis kettle after tetrahydrofuran is added, and stirred for 30 to 40 minutes, then the prepared cyclopentadienylsodium is added, back flowing of the liquid is kept under 60 to 70 DEG C, the stirring and reaction is kept for 1 to 2 hours, the reaction for preparing the catalyst is finished; after the backflow is finished, the solvent is recycled and heated to 65 to75 DEG C, when the liquid flow volume is reduced, the vacuum pressure distillation is started to recycle solvent, acetonitrile is continuously stirred and added when no liquid exists in the kettle, after being stirred for 1 hour under the room temperature, sodium chloride is removed to obtain the catalyst in mixture state. The invention has a simple reaction process, eliminates the demanding requirements and complexity of the synthesizing process for organic Co catalyst crystal.
Owner:HEBI SAIKE CHEM ENG CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com