Method for reducing pyridine ring to piperidine in 2-picoline-4-formic acid
A technology of picoline and pyridine ring, which is applied in the field of reduction of pyridine ring to piperidine, can solve the problems of complex process, expensive production raw materials, and low output, and achieve the effect of simple process, short reaction time and fast reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
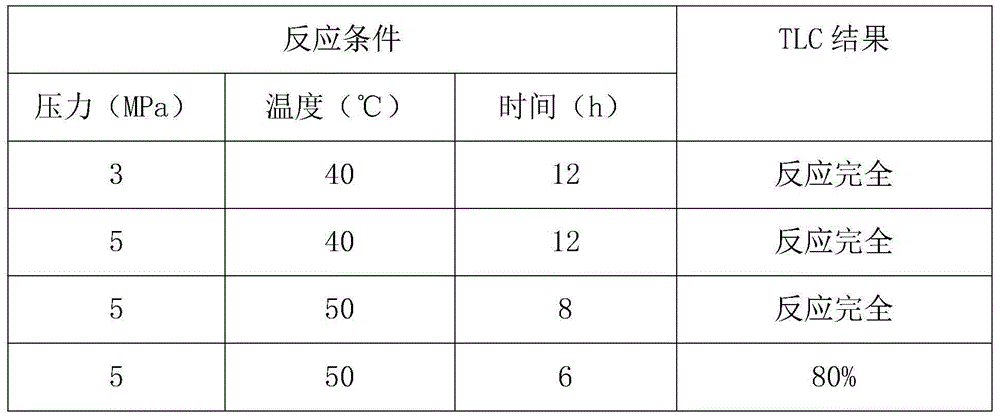
Examples
Embodiment Construction
[0017] The following clearly and completely describes the technical solutions in the embodiments of the present invention. Obviously, the described embodiments are only some of the embodiments of the present invention, but not all of them. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
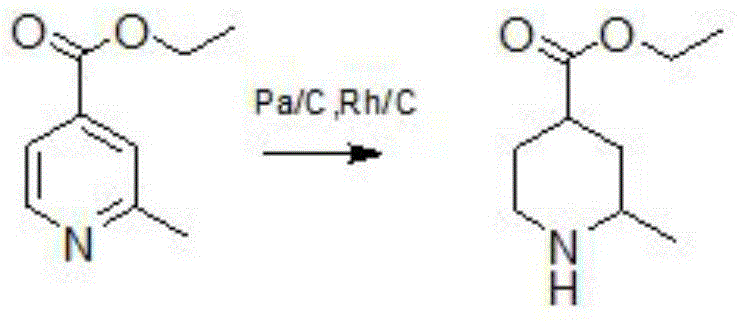
[0018] The reaction formula of reducing the pyridine ring into piperidine in 2-picoline-4-carboxylic acid is:
[0019]
[0020] A kind of method that pyridine ring is reduced into piperidine in 2-picoline-4-carboxylic acid, concrete steps are as follows,
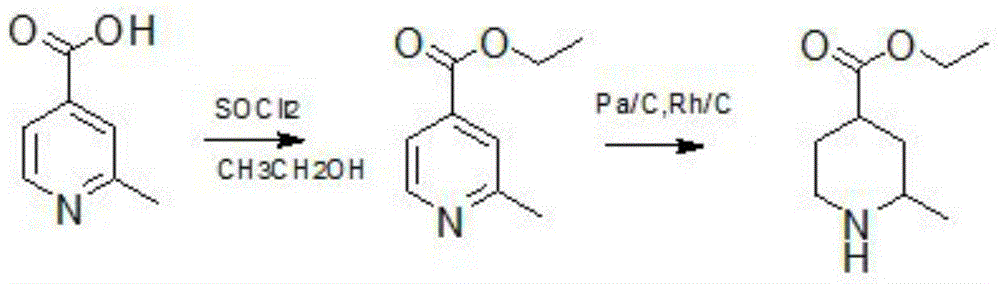
[0021] The first step prepares ethyl 2-methylpyridine-4-carboxylate, the preparation method is as follows,
[0022] First, dissolve 90-100 g of 2-picoline-4-carboxylic acid in 700 ml of ethanol solvent, stir evenly, place at 0°C, add 106 g of thionyl chloride dropwise, rise to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com