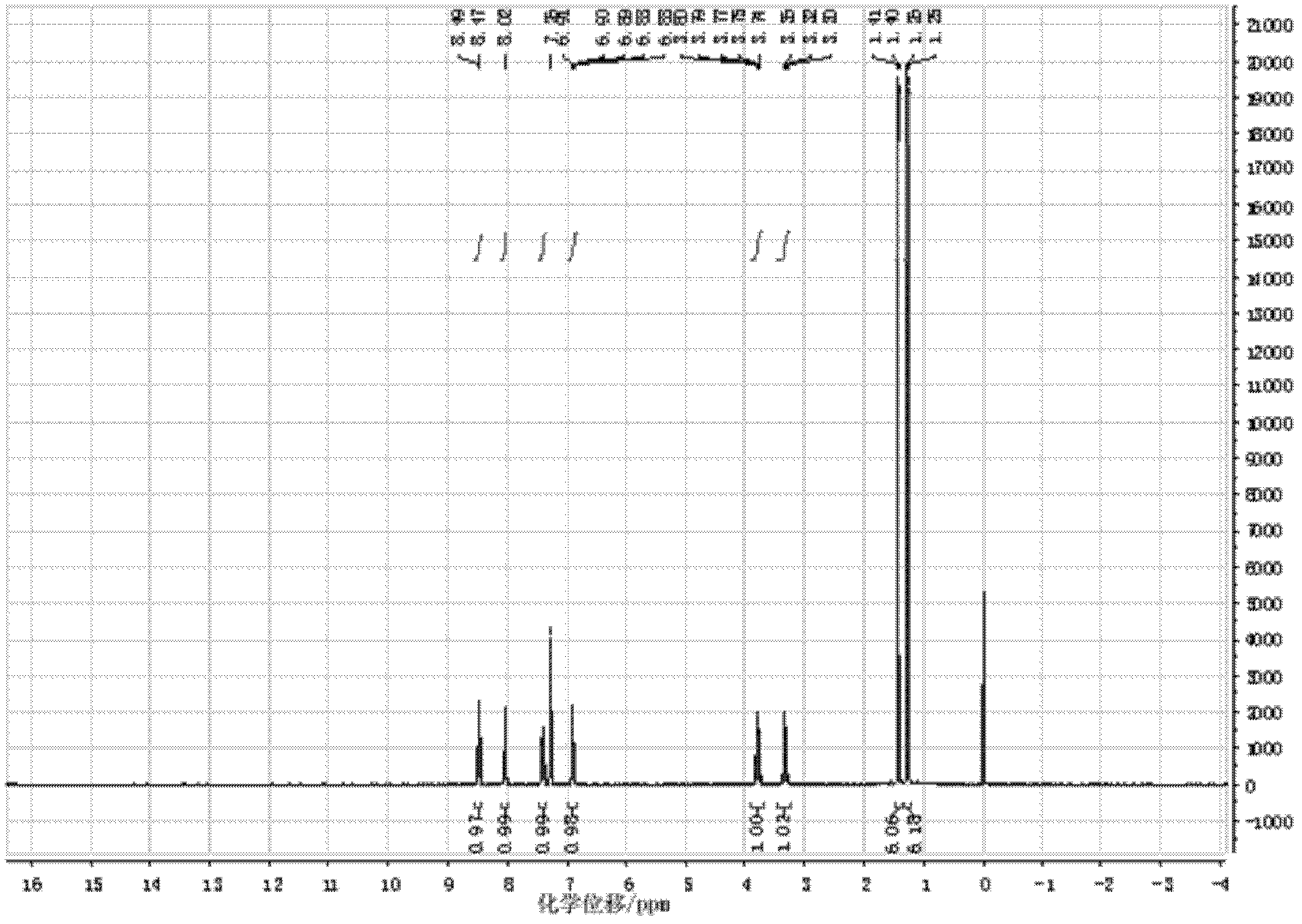
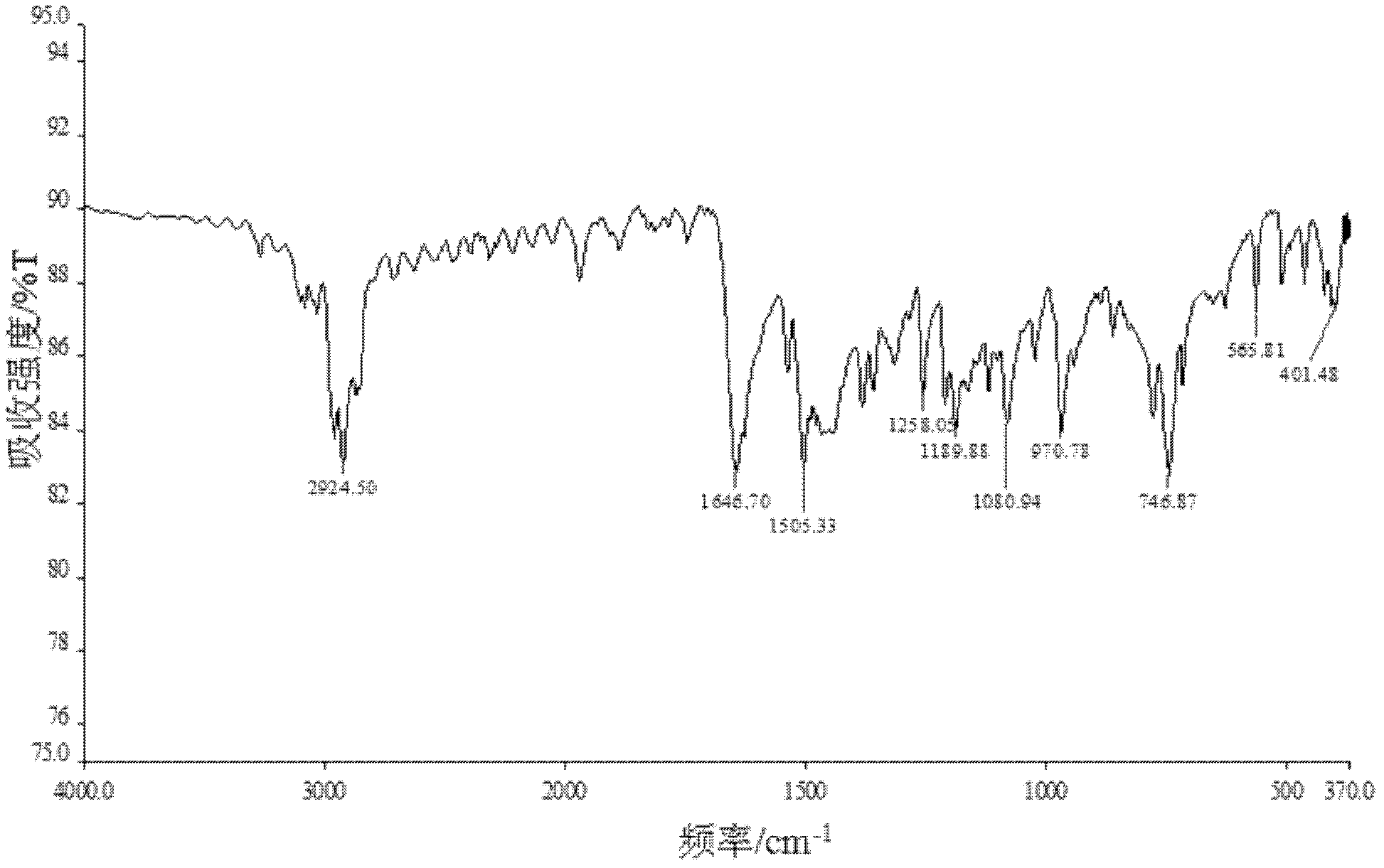
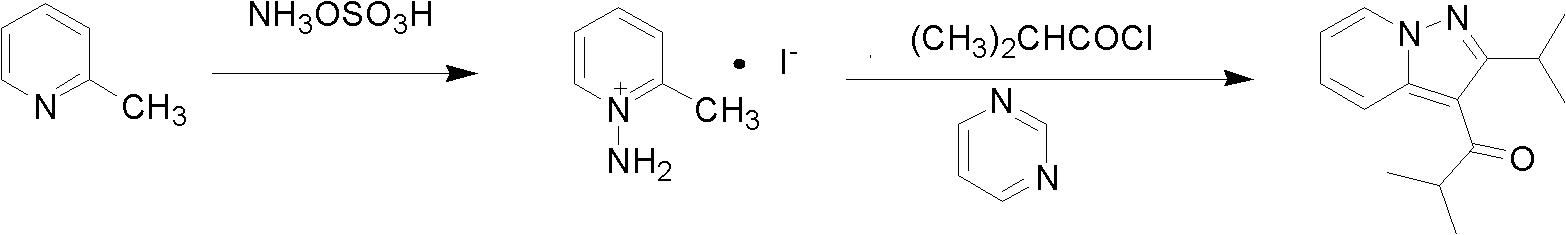
Preparation method of ibudilast
A technology of ibudilast and step 2, applied in the preparation field of ibudilast, can solve the problems of difficult operation, low yield, inability to realize large-scale industrial production, etc., and achieves high product purity, high yield, and reduced types and the effect of the quantity used
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0015] Specific embodiment one: present embodiment is a kind of preparation method of ibudilast, specifically completes according to the following steps:
[0016] 1. First, dissolve hydroxylamine oxysulfonic acid in the solvent, then add 2-picoline dropwise under ice-bath condition, after adding 2-picoline, raise the temperature to 50℃~90℃, and React at ~90°C for 0.5h~1h, then add inorganic base I, and stir at a stirring speed of 100r / min~300r / min until no bubbles are generated, then stir at a speed of 45r / min~90r / min and a temperature of 40 Rotary evaporation method at ℃~60℃ to constant weight, the obtained solid was washed 2 to 3 times with absolute ethanol, the filtrates obtained from the washing were combined, and the inorganic acid was added dropwise at the temperature of -15℃~-10℃ Or carry out acidification treatment with organic acid, the acidification treatment time is 0.5h~1h, and finally filter to obtain 1-amino-2-picoline acid compound;
[0017] 2. First, add 1-ami...
specific Embodiment approach 2
[0023] Specific embodiment two: the difference between this embodiment and specific embodiment one is: the solvent described in step one is selected from deionized water, methylene chloride, benzene, toluene and xylene. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0024] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is: the inorganic base I described in step one is potassium carbonate, sodium carbonate, sodium hydroxide or potassium hydroxide. Others are the same as in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com