A kind of original research quality ceftazidime and pharmaceutical preparation thereof
A technology for ceftazidime and ceftazidime side chain acid, which is applied in the field of ceftazidime and pharmaceutical preparations thereof, can solve the problems of low yield, intractable treatment and high production cost, and achieves the effects of reducing production cost, reducing the amount of waste residue and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
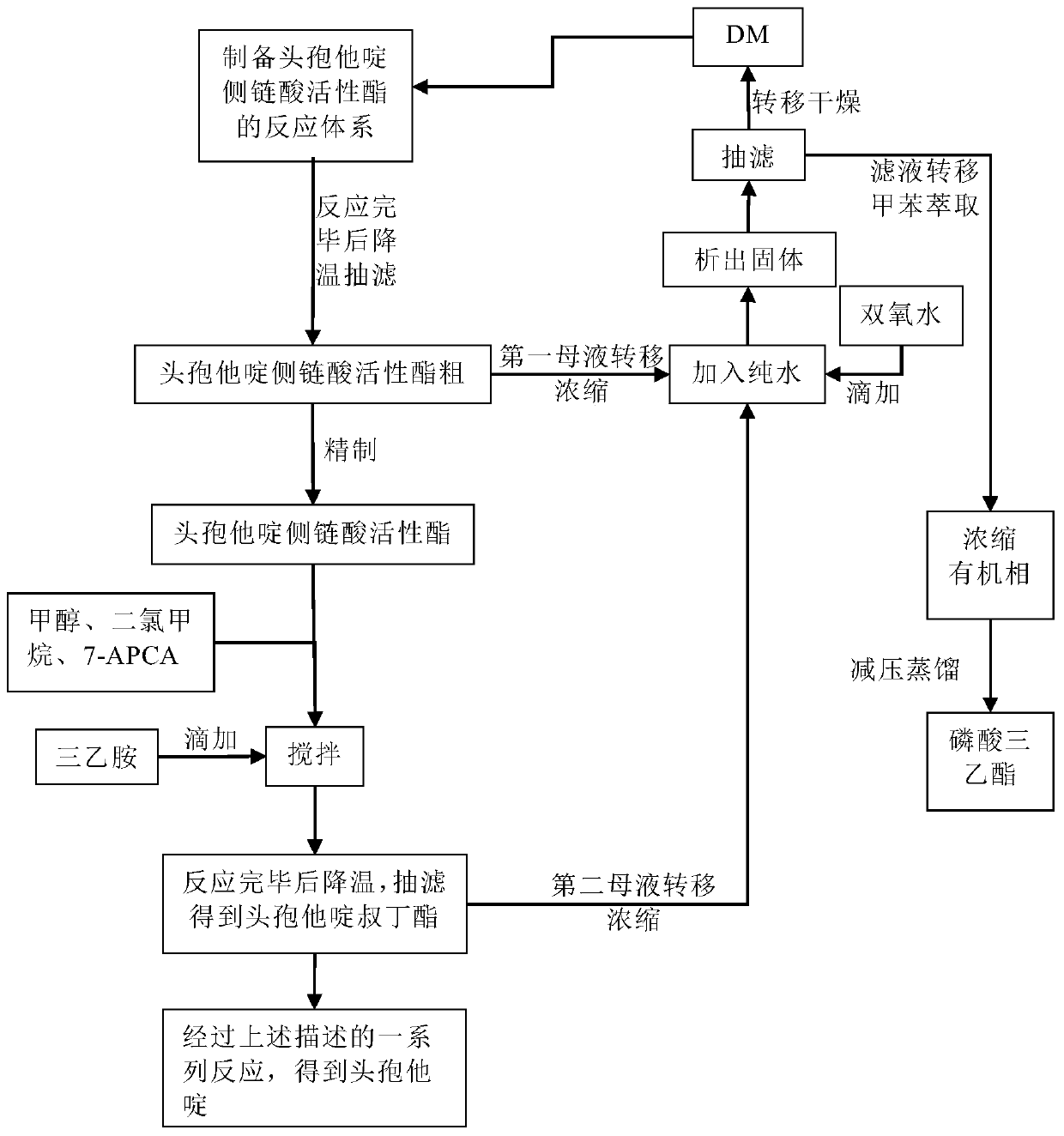
[0031] The preparation process flow diagram of ceftazidime provided by the invention is as follows figure 1 As shown, in a mixed solvent of toluene and acetonitrile, add ceftazidime side chain acid and dibenzothiazole disulfide, then add aniline, 2-picoline in turn, and then drop triethyl phosphite to establish the preparation of ceftazidime side chain The reaction system of the active ester is suction filtered after cooling down after the reaction is completed to obtain the crude product of the ceftazidime side chain acid active ester; the crude product is refined to obtain the ceftazidime side chain acid active ester, and the first mother liquor is recovered; In the mixed solvent, add ceftazidime side chain acid active ester and 7-APCA, add triethylamine dropwise during stirring, after the reaction is completed, cool down and crystallize, obtain ceftazime tert-butyl ester by suction filtration, and recycle the second mother liquor; ceftazidime tert-butyl The ester is subject...
Embodiment 1
[0035] Control the temperature around 20°C, add 30g of ceftazidime side chain acid and 30g of DM to the mixture of 100ml of toluene and 50ml of acetonitrile, add 8.0g of aniline, 0.4g of 2-methylpyridine in turn, and then dropwise add 4.5g of triethyl phosphite After the reaction is completed, the temperature is lowered, and the crude product is obtained after post-treatment, and the active ester of ceftazidime side chain acid is obtained after refining, with a yield of 95.1% and a purity of 99%. The first mother liquor of the crude product was recovered and concentrated to obtain 25 g of concentrated waste residue, No. 1.
[0036] Add 31.2g of ceftazidime side chain acid active ester and 25g of 7-APCA to 25ml of methanol and 100ml of dichloromethane mixed solvent under control of 0-10°C, add 12.5ml of triethylamine dropwise, after the heat preservation reaction is completed, filter to obtain ceftazidime tert-butyl Esters, yield 86.5%, purity 97.5%. The second mother liquor o...
Embodiment 2
[0039] Control the temperature around 20°C, add 30g of ceftazidime side chain acid and 30g of DM to the mixture of 100ml of toluene and 50ml of acetonitrile, add 8.0g of aniline, 0.4g of 2-methylpyridine in sequence, and then dropwise add 6.0g of triethyl phosphite After the reaction is completed, the temperature is lowered, and the crude product is obtained after post-treatment, and the active ester of ceftazidime side chain acid is obtained after refining, with a yield of 95.5% and a purity of 99%. The first mother liquor of the crude product was recovered and concentrated to obtain 25 g of concentrated waste residue, No. 1.
[0040] Add 31.2g of ceftazidime side chain acid active ester and 25g of 7-APCA to 25ml of methanol and 100ml of dichloromethane mixed solvent under control of 0-10°C, add 12.5ml of triethylamine dropwise, after the heat preservation reaction is completed, filter to obtain ceftazidime tert-butyl Esters, yield 87.1%, purity 97.9%. The second mother liqu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com