Preparation method of benzimidazole proton pump inhibitor intermediate
A proton pump inhibitor, benzimidazole type technology is applied in the field of preparation of benzimidazole type proton pump inhibitor intermediates, can solve the problems of cumbersome operation, low total yield and high cost, and achieves high product purity and high reaction efficiency. The effect of good yield and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
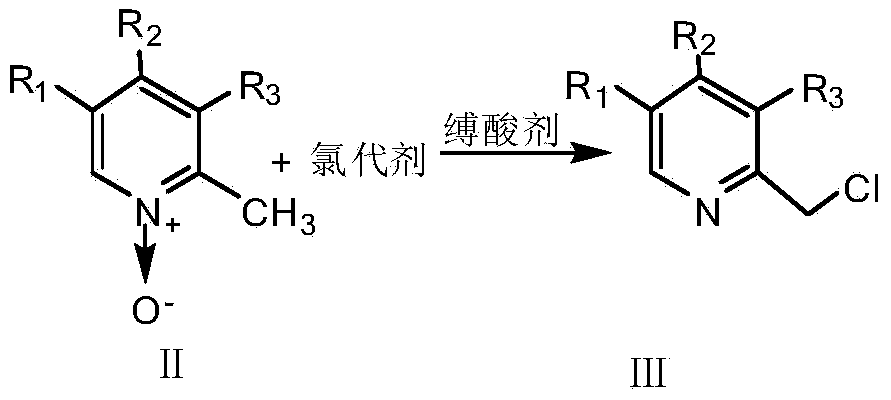
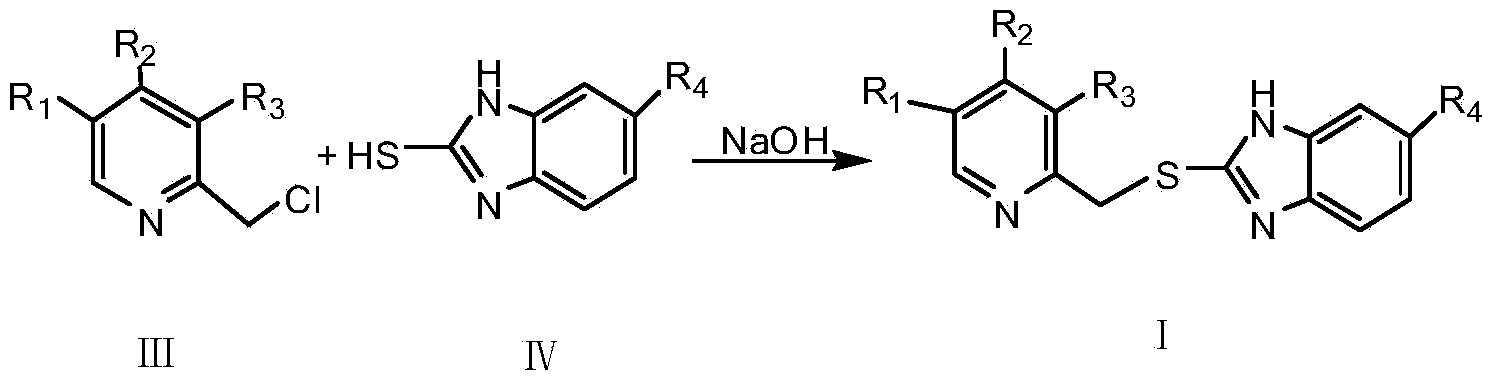
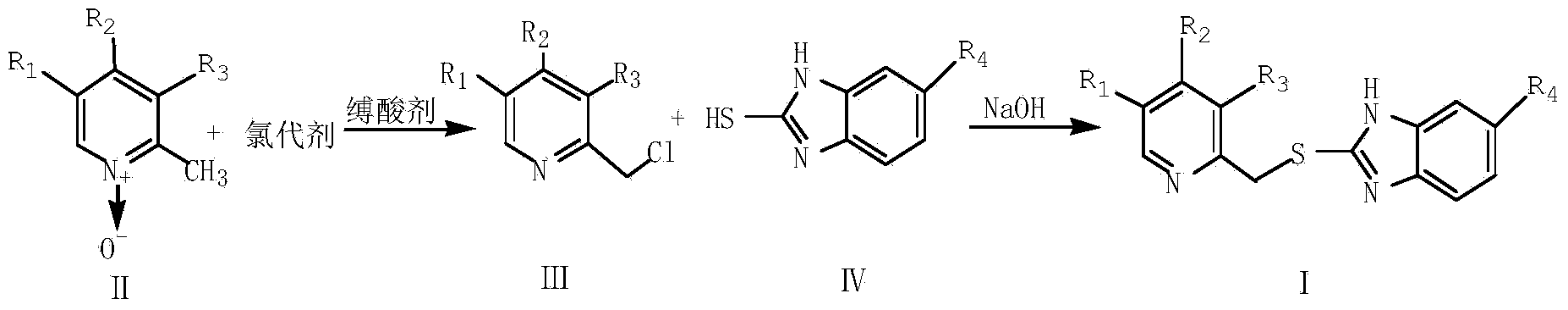
[0025] Synthesis of rabeprazole intermediate 2-{[4-(3-methoxypropoxy)-3-methylpyridin-2-yl]methylthio}-1H-benzimidazole
[0026] In a 250ml three-necked flask, dissolve 5.51g (0.026mol) of 4-(3-methoxypropoxy)-2,3-dimethyl-N-pyridine oxide in 25ml of benzene, and stir at a constant temperature of 80°C. Take 6.61g (0.043mol) of phosphorus oxychloride as a chlorination agent and 4.20g (0.041mol) of triethylamine as an acid-binding agent, and dilute to 20ml with dichloromethane respectively. After the dichloromethane solution of phosphorus oxychloride 2ml was added dropwise, the dichloromethane solution of triethylamine was added dropwise at the same rate. After 30 minutes, the dropwise addition was completed, and the reaction was stirred at a constant temperature for 2.5 hours. TLC detected that the reaction was complete, and the reaction was stopped. The pH was adjusted to 7 with saturated sodium carbonate solution, filtered, extracted with chloroform, dried, and the organic la...
Embodiment 2
[0029] Synthesis of omeprazole intermediate 5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylthio]-1H-benzimidazole
[0030] In a 250ml three-neck flask, dissolve 4.70g (0.028mol) of 4-methoxy-2,3,5-trimethyl-N-pyridine oxide in 25ml of benzene, and stir at a constant temperature of 75°C. Take 6.81g (0.044mol) of phosphorus oxychloride as a chlorination agent and 3.95g (0.039mol) of triethylamine as an acid-binding agent, and dilute to 25ml with dichloromethane respectively. After the dichloromethane solution of phosphorus oxychloride 3ml was added dropwise, the dichloromethane solution of triethylamine was added dropwise at the same rate. The dropwise addition was completed in about 30 minutes, and the reaction was performed at a constant temperature for 2 hours. TLC detected that the reaction was complete, and the reaction was stopped. The pH was adjusted to 7 with saturated sodium carbonate solution, filtered, extracted with chloroform, dried, and the organic layer wa...
Embodiment 3
[0033]Synthesis of lansoprazole intermediate 2-{[4-(2,2,2-trifluoromethoxy)-3-methylpyridin-2-yl]methylthio}-1H-benzimidazole
[0034] Take 6.64g (0.03mol) of 4-(2,2,2-trifluoroethoxy)-2,3-dimethyl-N-pyridine oxide in a 250ml three-necked flask, add 25ml of benzene, and stir at a constant temperature of 90°C . Take 7.34g (0.048mol) of phosphorus oxychloride as a chlorination agent and 4.24g (0.042mol) of triethylamine as an acid-binding agent, and dilute to 25ml with dichloromethane respectively. After the dichloromethane solution of phosphorus oxychloride 3ml was added dropwise, the dichloromethane solution of triethylamine was added dropwise at the same rate. After about 40 minutes of dropwise addition, continue to stir and react for 3 hours, TLC detects that the reaction is complete, stop the reaction, adjust the pH to 7 with saturated sodium carbonate solution, filter, extract with chloroform, dry, and evaporate the organic layer to obtain 2-chloromethyl- 4-(2,2,2-Triflu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com