Triamine monomer containing pyridine structure and preparation method thereof
A technology of triamine monomer and pyridine, which is applied in the field of triamine monomer containing pyridine structure and its preparation, and achieves the effects of high permeability, low reaction temperature and reasonable selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
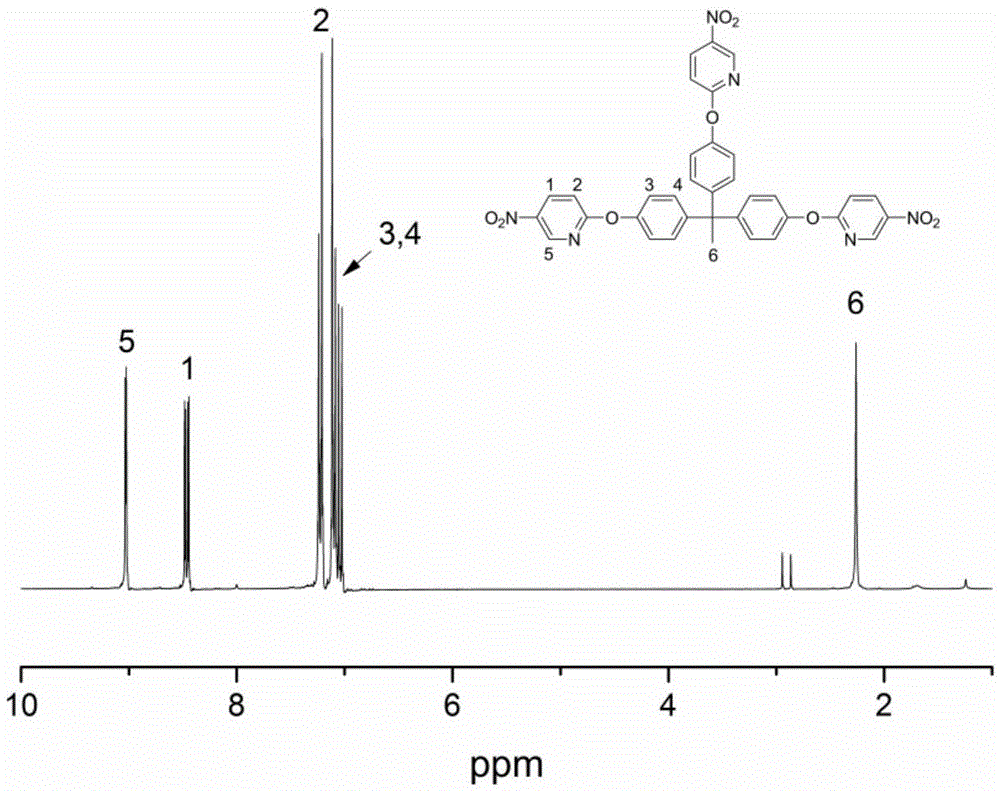
[0022] (1) Add 1,1,1-tris(4-hydroxyphenyl)ethane (10g, 32.64mmol) and 180mL N,N'-dimethylformamide into a three-necked flask, control the temperature at 10°C, and add potassium carbonate ( 18.04 g, 130.56 mmol), 2-chloro-5-nitropyridine (20.70 g, 130.56 mmol). The reaction was slowly heated to 60°C for 8 hours.
[0023] (2) After cooling down to room temperature, the reaction solution was poured into deionized water, a large amount of solids were precipitated, filtered, and washed with deionized water for 3 times, and the crude product was recrystallized with N,N'-dimethylformamide / water, 19 g of product after recrystallization.
[0024] (3) The recrystallized solid product was vacuum treated at 80° C. for 8 hours.
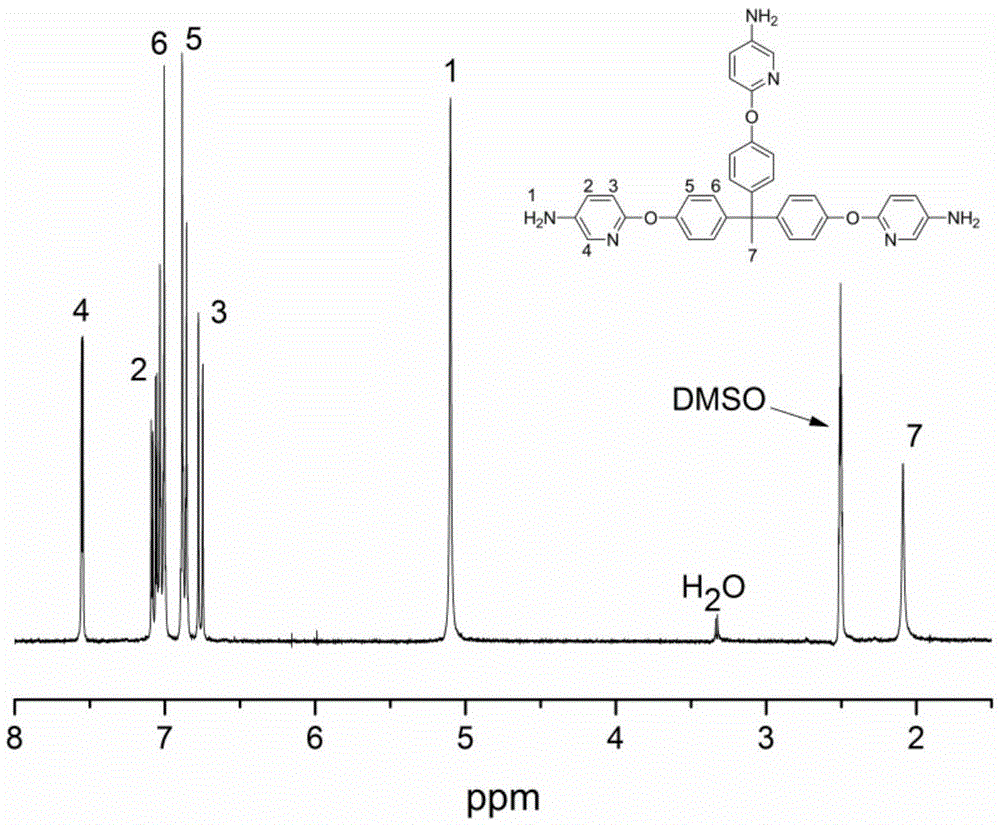
[0025] (4) Add 19 g of the intermediate product after recrystallization to a three-necked flask containing 200 mL of dioxane under nitrogen protection, add 3.8 g of palladium carbon, heat the reaction solution to 80 ° C and add 40 mL of hydrazine hydrate dropwis...
Embodiment 2
[0030] (1) Add 1,1,1-tris(4-hydroxyphenyl)ethane (5g, 16.48mmol) and 180mL N-methylpyrrolidone into a three-necked flask, control the temperature at 20°C, add sodium carbonate (8.73g, 82.40mmol ), 2-chloro-5-nitropyridine (13.06 g, 82.40 mmol). The reaction was slowly heated to 90°C for 5 hours.
[0031] (2) After cooling down to room temperature, the reaction solution was poured into deionized water, a large amount of solids were precipitated, filtered, and washed with deionized water three times. The crude product was recrystallized with N-methylpyrrolidone / water, and the product after recrystallization was 10 g.
[0032] (3) The recrystallized solid product was vacuum treated at 80° C. for 8 hours.
[0033] (4) Add 10 g of the intermediate product after recrystallization to a three-necked flask containing 200 mL of ethanol under nitrogen protection, add 3 g of platinum carbon, heat the reaction solution to 80 ° C and add 15 mL of hydrazine hydrate dropwise, and control th...
Embodiment 3
[0035] Add 1,1,1-tris(4-hydroxyphenyl)ethane (10g, 32.64mmol) and 150mL N,N'-dimethylformamide into the three-necked flask, control the temperature at 10°C, add sodium hydroxide (7.83g , 195.84mmol), 2-chloro-5-nitropyridine (15.52g, 97.92mmol). The reaction was slowly heated to 30°C for 5 hours. The reaction solution was poured into deionized water, a large amount of solid was precipitated, filtered, and washed with deionized water three times. The crude product was recrystallized from N,N'-dimethylformamide / water and vacuum treated at 60°C for 8 hours. Add 18g of the intermediate product after recrystallization to a three-necked flask containing 200mL of methanol under nitrogen protection, add 3g of platinum carbon, heat the reaction solution to 80°C, add 40mL of hydrazine hydrate dropwise, and control the reaction within 1 hour to complete the dropwise addition, at 80°C After reacting for 6 hours, it was filtered hot, and the filtrate was distilled under reduced pressure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com