Lanthanum calcium iron cobalt calcium titanium ore type catalyst for oxidizing and reforming ethanol and method for preparing catalyst
A perovskite type, ethanol oxidation technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve accelerated catalysts, unseen, unfavorable And other problems, to achieve good stability, high anti-carbon performance, high stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The catalyst preparation process is as follows: weigh La(NO 3 ) 3 ·6H 2 O 5.116g, Ca(NO 3 ) 2 4H 2 O 0.307g, Fe(NO 3 ) 3 9H 2 O 4.749g, Co(NO 3 ) 2 ·6H 2 O 0.378g, citric acid 6.521g, polyethylene glycol 400 0.5g, join in the deionized water of 250ml and make solution, stir in the water bath of 85 ℃, stirring rate is 120N / min, evaporate and concentrate to obtain viscous solid, Then dry at 120°C for 24 hours to obtain a foamy solid. Roast in a muffle furnace, heat up to 450°C at a heating rate of 5°C / min for 2 hours, and then heat up to 750°C at a heating rate of 5°C / min Roasted for 6 hours to obtain La 0.9 Ca 0.1 Fe 0.9 co 0.1 o 3 Catalyst 2.783g, yield is 92%.
[0018] The catalyst prepared above is used for the oxidative reforming of ethanol to prepare hydrogen-rich gas:
[0019] The performance test of the catalyst was carried out on a miniature fixed-bed reactor, and the dosage of the catalyst was 75 mg, 40-60 mesh. Use N before reacting 2 Purge f...
Embodiment 2
[0022] The catalyst preparation process is as follows: weigh La(NO 3 ) 3 ·6H 2 O 4.743g, Ca(NO 3 ) 2 4H 2 O 0.640g, Fe(NO 3 ) 3 9H 2 O 4.402g, Co(NO 3 ) 2 ·6H 2 O 0.789g, citric acid 6.800g, polyethylene glycol 400 0.5g, were added to 250ml of deionized water to form a solution, stirred in a water bath at 85°C, the stirring rate was 120N / min, evaporated and concentrated to obtain a viscous solid, Then dry at 120°C for 24 hours to obtain a foamy solid. Roast in a muffle furnace, heat up to 450°C at a heating rate of 5°C / min for 2 hours, and then heat up to 750°C at a heating rate of 5°C / min Roasted for 6 hours to obtain La 0.8 Ca 0.2 Fe 0.8 co 0.2 o 3 Catalyst 2.835g, yield is 94%.
[0023] The catalyst prepared above was used for oxidative reforming of ethanol to prepare hydrogen-rich gas, and the reforming conditions were the same as in Example 1. The test results are as follows: La 0.8 Ca 0.2 Fe 0.8 co 0.2 o 3 The catalyst completely converted ethanol a...
Embodiment 3
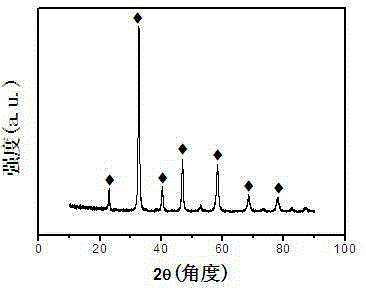
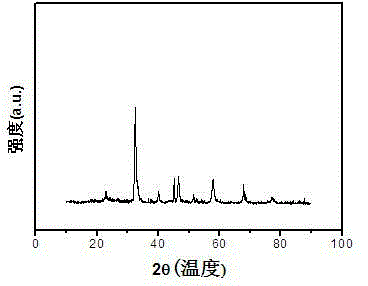
[0025] The catalyst preparation process is as follows: weigh La(NO 3 ) 3 ·6H 2 O 4.335g, Ca(NO 3 ) 2 4H 2 O 1.003g, Fe(NO 3 ) 3 9H 2 O 4.024g, Co(NO 3 ) 2 ·6H 2 O 1.236g, citric acid 7.105g, polyethylene glycol 400 0.5g, were added to 250ml of deionized water to form a solution, stirred in a water bath at 85°C, the stirring speed was 120N / min, evaporated and concentrated to obtain a viscous solid, Then dry at 120°C for 24 hours to obtain a foamy solid. Roast in a muffle furnace, heat up to 450°C at a heating rate of 5°C / min for 2 hours, and then heat up to 750°C at a heating rate of 5°C / min Roasted for 6 hours to obtain La 0.7 Ca 0.3 Fe 0.7 co 0.3 o 3 Catalyst 2.895g, yield is 96%, and its chemical formula XRD diffraction pattern is as figure 1 shown.
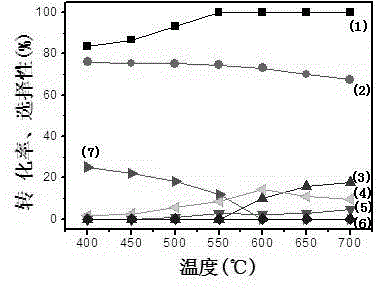
[0026] The catalyst prepared above was used for oxidative reforming of ethanol to prepare hydrogen-rich gas, and the reforming conditions were the same as in Example 1. The test results are as follows: La 0.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com