Method for purifying hexachlorocyclopentadiene
A technology for hexachlorocyclopentadiene and a purification method, which is applied in chemical instruments and methods, halogenated hydrocarbon preparation, organic chemistry and other directions, can solve the difficulty of separating crude hexachlorocyclopentadiene products and the inability to obtain high-purity hexachlorocyclopentadiene. cyclopentadiene, large amount of solvent consumption, etc., to avoid product loss, easy operation, and improved economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
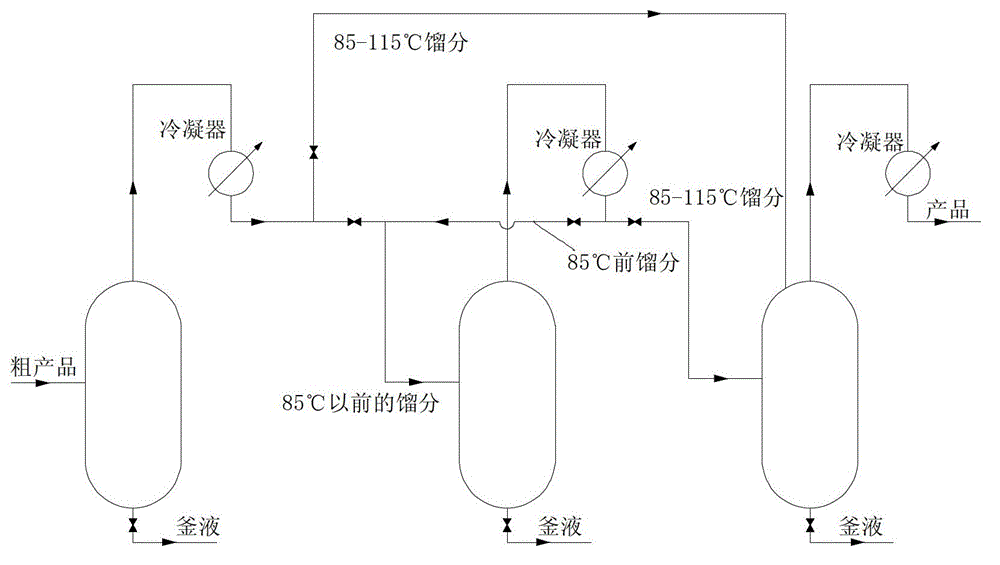
[0030] Weigh 150g of crude hexachlorocyclopentadiene, distill the above liquid under reduced pressure (vacuum degree is 10 mmHg), collect the fraction before 76°C and the fraction at 76-116°C respectively, and then distill the fraction before 76°C under 100°C Stir at constant temperature for 3 hours, distill, collect the fraction at 76-116°C, mix the fractions at 76-116°C obtained from the two distillations, stir at constant temperature at 100°C for 3 hours, then distill under reduced pressure (vacuum degree is 10 mmHg), collect 105 85 g of distillate at -112°C is high-purity hexachlorocyclopentadiene with a purity of 98.7% and a yield of 93.1%.
Embodiment 2
[0032] Weigh 800g of crude hexachlorocyclopentadiene, add 5g of anhydrous magnesium sulfate to remove the contained water, let stand overnight, and filter with suction to obtain a clear oily liquid. Distill the above liquid under reduced pressure (vacuum degree is 14 mmHg), collect the fraction before 78°C and the fraction at 78-117°C respectively, then stir the fraction before 78°C at 100°C for 3.5 hours, and collect 78-117°C by vacuum distillation. For the fraction at 117°C, continue to stir the fraction at 78-117°C obtained by two distillations at 100°C for 3 hours, then distill under reduced pressure (vacuum degree is 14 mmHg), collect 449g of fraction at 106-113°C, which is high-purity six Chlorocyclopentadiene, the purity is 99.2%, and the yield is 92.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com