Application of curcumin solid lipid nano-particle serving as medicament for treating asthma
A technology of solid lipid nano and curcumin, which is applied in drug combination, liposome delivery, respiratory diseases, etc., can solve the problems that have not been reported, and achieve simple technical operation, stable reaction system, and improved bioavailability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Weigh 40 mg of stearic acid, 20 mg of lecithin, and 40 mg of curcumin, dissolve in 10 ml of chloroform;
[0042] Use a 10# needle to inject the organic phase into 30ml of the water phase that has been preheated to 75°C containing 30mg Myrj53 at a constant speed, and stir at 1200rpm for about 1 hour. Ionized water, continue to stir at 1200rpm at room temperature for 2 hours, fully centrifuge to collect samples, wash twice with PBS, and freeze-dry to obtain the finished drug.
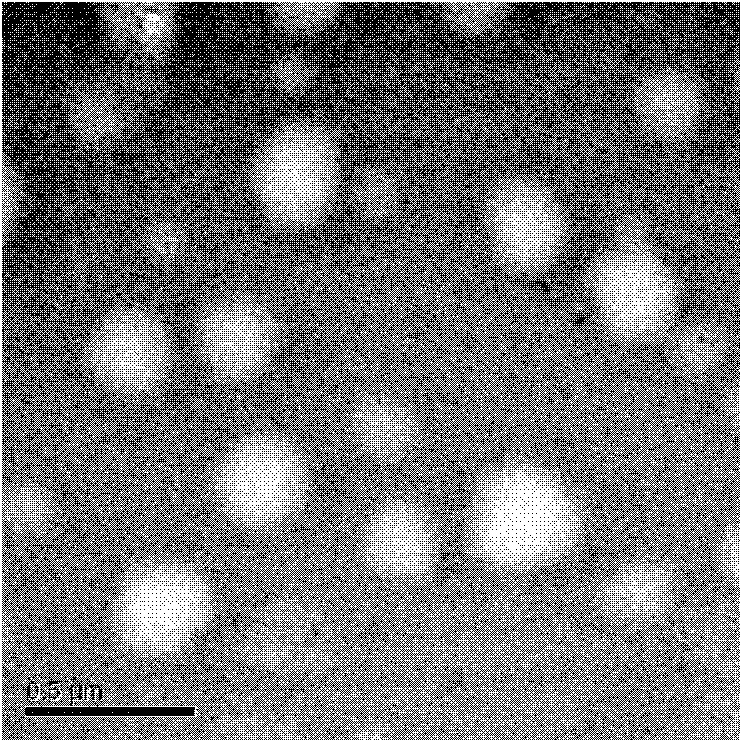
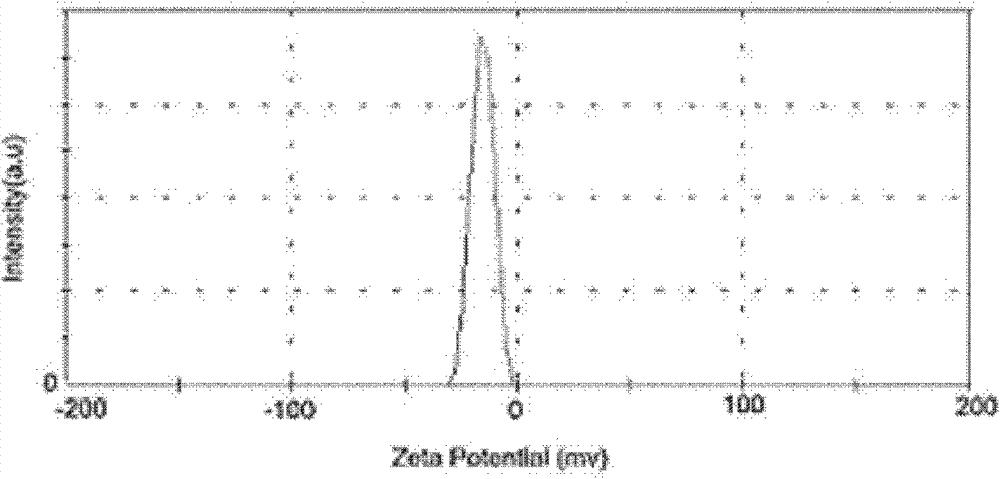
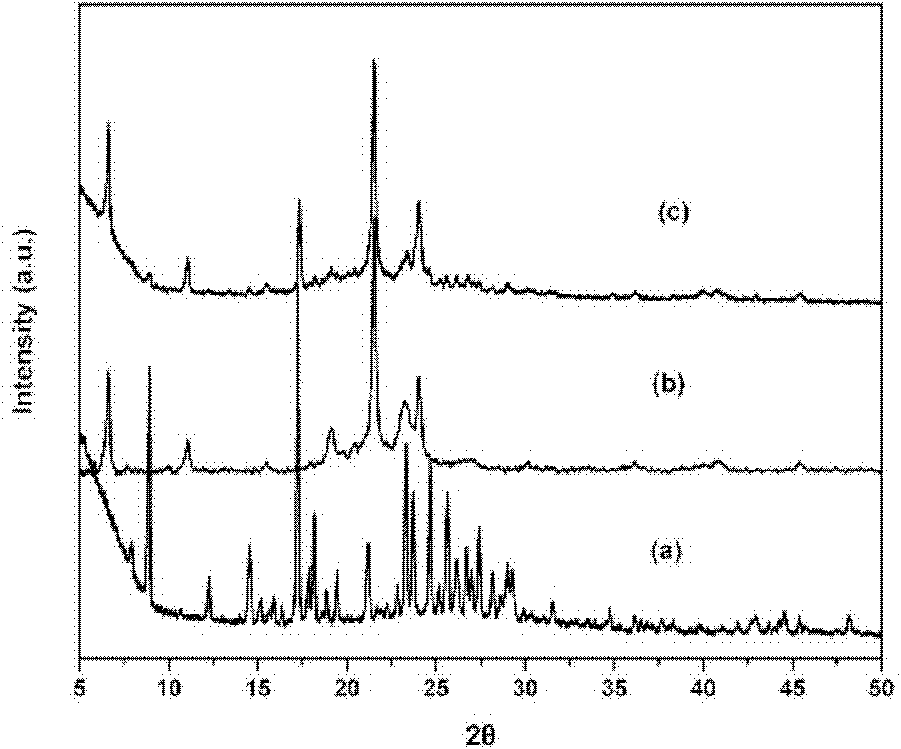
[0043] The particle size of SLN-curcumin is 50-200nm, which is circular (such as figure 1 ), the surface is negatively charged, and the surface potential is -20mV (such as figure 2 ). Show that SLN-curcumin has all characteristic peaks of SLN by XRD analysis, has the part characteristic peak of curcumin simultaneously, shows that SLN wraps curcumin in lipid, and the two are organically combined (such as image 3 ).
[0044] The drug release experiment of SLN-curcumin was carried out in vitro b...
Embodiment 2
[0047] Weigh 40 mg of stearic acid, 20 mg of lecithin, and 20 mg of curcumin, and dissolve them in 10 ml of chloroform; inject the organic phase into 30 ml of the aqueous phase containing 30 mg of Myrj53 at a constant speed with a 10# needle, stir at 75 °C and 1200 rpm for about 1 hour, and wait until the system About 5ml remained, removed the water bath, quickly poured in the prepared 4°C deionized water, continued to stir at 1200rpm at room temperature for 2 hours, centrifuged fully to collect the sample, washed twice with PBS, and freeze-dried to obtain the finished drug.
[0048] The particle size of SLN-curcumin is 50-200nm, round in shape, negatively charged on the surface, and the surface potential is -25mV. In the PBS solution of 1% Tween80, the drug release of SLN-curcumin can reach more than 7 days, and the drug release can reach 45% in about 2 days.
[0049] In the blood drug concentration experiment, a total of 3 groups of BALB / C mice were used, 3 in each group, wh...
Embodiment 3
[0052] Weigh 20 mg of stearic acid, 10 mg of lecithin, and 15 mg of curcumin, and dissolve them in 10 ml of chloroform; inject the organic phase into 30 ml of the aqueous phase containing 20 mg of Myrj53 at a constant speed with a 10# needle, stir at 75 °C and 1200 rpm for about 2 hours, and wait until the system About 5ml remained, removed the water bath, quickly poured in the prepared 4°C deionized water, continued to stir at 1200rpm at room temperature for 2 hours, centrifuged fully to collect the sample, washed twice with PBS, and freeze-dried to obtain the finished drug.
[0053] The particle size of SLN-curcumin is 50-200nm, round in shape, negatively charged on the surface, and the surface potential is -25mV. In the PBS solution of 1% Tween80, the drug release of SLN-curcumin can reach more than 5 days, and the drug release can reach 48% in about 2 days.
[0054] In the blood drug concentration experiment, a total of 3 groups of BALB / C mice were used, 3 in each group, w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com