Preparation method and products of danofloxacin mesylate liposome
The technology of danfloxacin mesylate and danfloxacin mesylate is applied in the fields of preparation of danfloxacin mesylate lung-targeted liposome and preparation of veterinary drugs, and can solve the production process Complex, expensive, liver toxicity and other problems, to achieve the effect of good dispersion, good stability, long efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of embodiment 1 dafloxacin mesylate liposome of the present invention
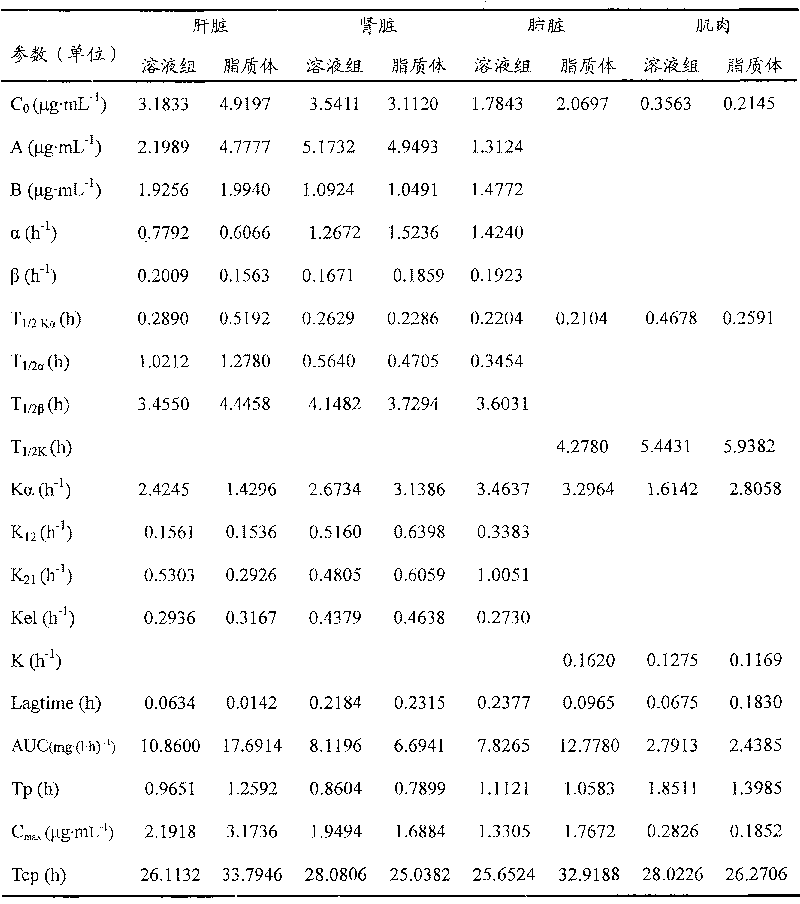
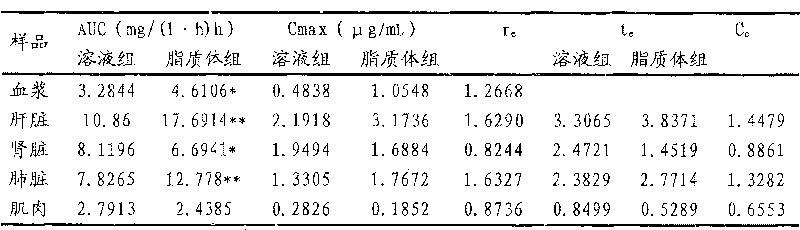
[0023] Egg yolk lecithin (EPC) and cholesterol (CH) with a weight ratio of 3:2 and 2.6% stearylamide (SA) of the two weights were thoroughly mixed, dissolved with anhydrous ether, and then evaporated under reduced pressure on a rotary basis. Remove the ether to obtain a uniform translucent phospholipid film. After vacuum drying for 24 hours, add 250mmol / L ammonium sulfate solution to elute. The resulting blank liposomes are sized through a 3μm microporous filter membrane and dialyzed 3 times with 150mmol / L NaCl solution at room temperature. , 24 hours each time; take the blank liposomes after dialysis, add 10mg / mL dafloxacin mesylate solution, the ratio of drug to lipid is 2:5, adjust the pH value with 100mmol / L NaOH solution, and then place it at 35 Incubate in a water bath at ℃ for 15 minutes, quantify with PBS solution, and use the freeze-thaw method to filter the suspension with ...
Embodiment 2
[0025] The preparation of embodiment 2 dafloxacin mesylate liposomes of the present invention
[0026] Egg yolk lecithin (EPC) and cholesterol (CH) with a weight ratio of 4:3 and 2.8% stearylamide (SA) of the two weights were thoroughly mixed, dissolved in anhydrous ether, and then evaporated under reduced pressure on a rotary basis. Remove the ether to obtain a uniform translucent phospholipid film. After vacuum drying for 32 hours, add 280mmol / L ammonium sulfate solution to elute. The obtained blank liposomes are sized through a 3μm microporous filter membrane and dialyzed 3 times with 150mmol / L NaCl solution at room temperature. , each time for 32 hours; take the blank liposome after dialysis, add 10mg / mL dafloxacin mesylate liquid, the ratio of drug to lipid is 2:6, adjust the pH value with 100mmol / L NaOH solution, and then place it at 37 Incubate in a water bath at ℃ for 12 minutes, quantify with PBS solution, and use the freeze-thaw method to filter the suspension with a...
Embodiment 3
[0028] Embodiment 3 The preparation of dafloxacin mesylate liposome of the present invention
[0029]Egg yolk lecithin (EPC) and cholesterol (CH) with a weight ratio of 3:2.5 and stearamide (SA) of 3.0% by weight of the two were thoroughly mixed, dissolved in anhydrous ether, and evaporated under reduced pressure on a rotary basis. Remove the ether to obtain a uniform translucent phospholipid film. After vacuum drying for 24 hours, add 200mmol / L ammonium sulfate solution to elute. The resulting blank liposomes are sized through a 3μm microporous filter membrane and dialyzed 3 times with 150mmol / L NaCl solution at room temperature. , 24 hours each time; take the blank liposome after dialysis, add 10mg / mL dafloxacin mesylate liquid, the ratio of drug to lipid is 2:3, adjust the pH value with 100mmol / L NaOH solution, and then place it at 35 Incubate in a water bath at ℃ for 15 minutes, quantify with PBS solution, and use the freeze-thaw method to filter the suspension with a 3 μm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com