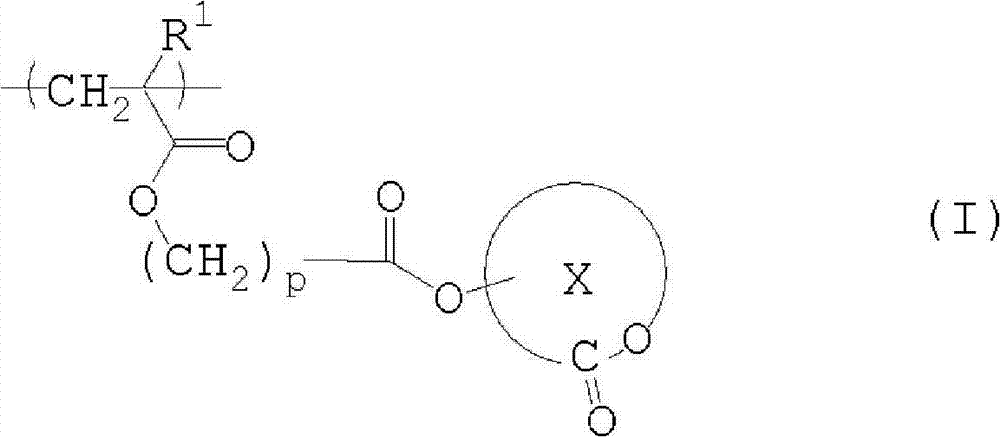
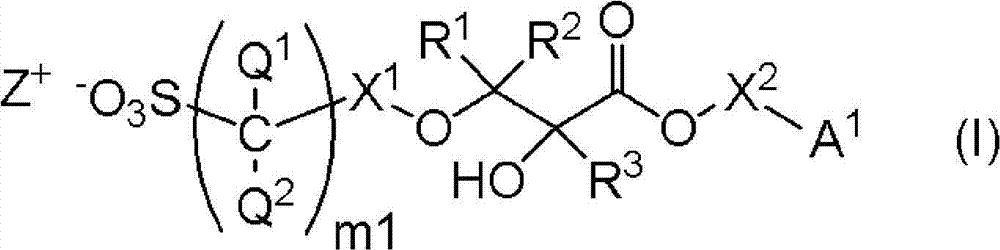
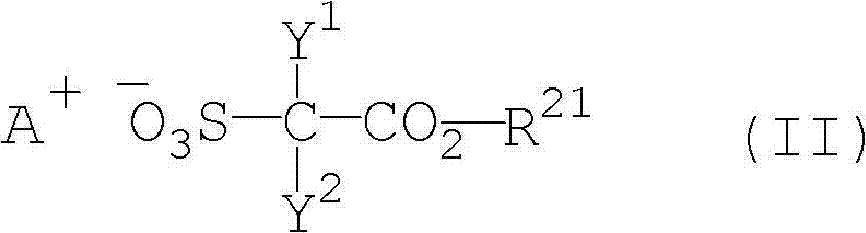Salt, photoresist composition and method for producing photoresist pattern
A technology of photoresist and composition, applied in the field of salt and photoresist composition, to achieve the effect of improving exposure latitude
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0341]
[0342] In a mixture of 10 parts of a compound represented by formula (I-1-a) produced by OSAKAORGANICCEHMICALINDUSTRIES, Ltd. and 30 parts of chloroform, 11.3 parts of 50% 4-methylmorpholine N-oxide aqueous solution and 0.78 parts of a 4% aqueous solution of osmium tetroxide, and the resulting mixture was stirred at room temperature for 24 hours. To the reaction mixture was added 82 parts of a 5% aqueous oxalic acid solution, followed by extraction with chloroform, followed by separation into a chloroform layer. After the chloroform layer was washed with deionized water, it was concentrated under reduced pressure to obtain 13.22 parts of the compound represented by the formula (I-1-b).
[0343] 1 H-NMR (DMSO-d 6 ): δ=4.8-4.7(1H, s), 4.7-4.6(1H, m), 3.5-3.3(2H, m), 2.2-1.9(9H, m), 1.7-1.5(6H, m), 1.1 (3H, s)
[0344] To a mixture of 6 parts of the compound represented by the formula (I-1-c) (the compound was produced by the method described in JP2008-127367A) an...
Embodiment 2
[0349]
[0350] To a mixture of 10 parts of a compound represented by formula (I-6-a) produced by Idemitsu Kosan Co., Ltd. and 30 parts of chloroform, 10.7 parts of 50% 4-methylmorpholine N-oxide solution and 0.73 parts of 4% osmium tetroxide in water. The resulting mixture was stirred at room temperature for 24 hours. To the resulting reaction mixture, 77 parts of a 5% aqueous oxalic acid solution was added, and extracted with chloroform, followed by separation into a chloroform layer. The chloroform layer was washed with deionized water, after which it was concentrated under reduced pressure to obtain 13.92 parts of the compound represented by formula (I-6-b).
[0351] 1 H-NMR (DMSO-d 6 ): δ=5.0-4.8 (1H, m), 4.8-4.6 (1H, m), 3.5-3.2 (2H, m), 2.5-2.4 (2H, m), 2.3-2.2 (7H, m), 2.0 -1.7(4H, m), 1.1(3H, s)
[0352] To a mixture of 6 parts of the compound represented by formula (I-6-c) and 30 parts of chloroform, 2.33 parts of 1,1'-carbonyldiimidazole was added, after whi...
Embodiment 3
[0357]
[0358] To a mixture of 10 parts of the compound represented by the formula (I-20-a) produced by Idemitsu Kosan Co., Ltd. and 30 parts of chloroform, 9.5 parts of 50% 4-methylmorpholine N-oxide solution and 0.65 parts of 4% osmium tetroxide in water. The resulting mixture was stirred at room temperature for 24 hours. To the resulting reaction mixture, 70 parts of a 5% aqueous oxalic acid solution was added, followed by extraction with chloroform, followed by separation into a chloroform layer. After the chloroform layer was washed with deionized water, it was concentrated under reduced pressure to obtain 11.53 parts of the compound represented by the formula (I-20-b).
[0359] 1 H-NMR (DMSO-d 6 ): δ=5.0-4.2 (1H, m), 3.6-3.4 (2H, m), 2.6-2.5 (2H, m), 2.5-2.4 (1H, m), 2.1-1.4 (12H, m), 1.2 (3H, s), 1.0-0.9 (6H, m)
[0360] To a mixture of 6 parts of the compound represented by formula (I-20-c) and 30 parts of chloroform, 2.33 parts of 1,1'-carbonyldiimidazole was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com