Iloperidone impurity and its preparation method
A technology for iloperidone and impurities, which is applied in the testing of iloperidone and its impurities, the impurity of iloperidone and its preparation, and can solve the problems of low product purity and lack of single impurity control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
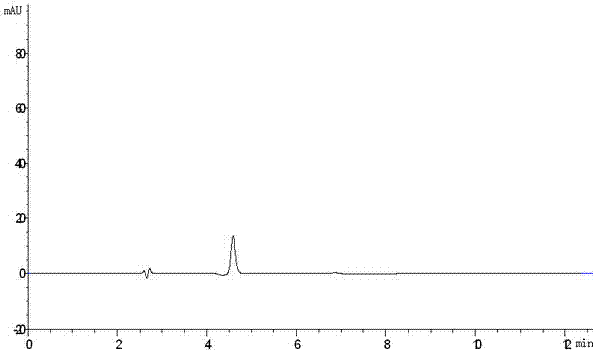
[0078] Embodiment 1: the HPLC analysis method of iloperidone
[0079] Get an appropriate amount of iloperidone, add an appropriate amount of acetonitrile for sonication to dissolve, dilute with mobile phase to make a solution containing about 0.3 mg per 1 ml, as the test solution, according to the high performance liquid chromatography (Chinese Pharmacopoeia 2010 version two Appendix VD) Determination. Octadecylsilane bonded silica gel is used as filler, ammonium acetate solution (take 5.0g of ammonium acetate, add 1000ml of water to dissolve, shake well)-acetonitrile (volume ratio 50:50) as mobile phase, and the detection wavelength is 230nm. The number of theoretical plates should not be less than 2000 based on the calculation of iloperidone. Precisely measure 20 μl of the test solution, inject it into a liquid chromatograph, record the chromatogram to 6 times the retention time of the main peak, and calculate the purity of iloperidone with the area normalization method. I...
Embodiment 2
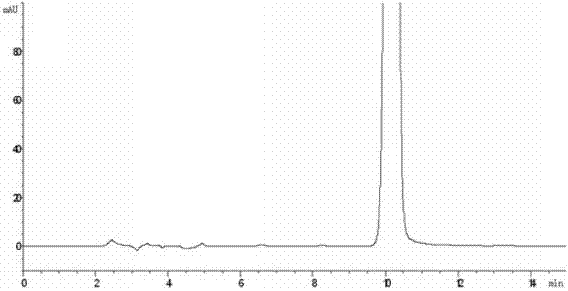
[0081] Embodiment 2: the preparation of formula I compound
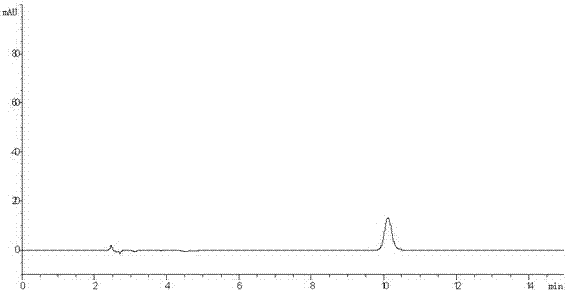
[0082] Suspend 10g of iloperidone in 500mL of acetonitrile, add 5ml of 30% hydrogen peroxide dropwise, and react at room temperature for 24h. The reaction solution was extracted twice with dichloromethane, the combined dichloromethane layers were washed with water, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and the residue was purified with acetonitrile to obtain 1-(3-(4-acetyl- 2-methoxyphenoxy)propyl)-4-(6-fluoro-1,2-benzisoxazol-3-yl)piperidine-N-oxide (compound of formula I). HPLC purity 99.1%.
[0083] MH in mass spectrum [ESI-MS, m / z] + Peak is 443.
[0084] 13C NMR (400MHz, DMSO) δ (ppm): 196.3, 164.6, 163.1, 160.7, 152.3, 148.6, 129.9, 123.6, 123.0, 117.0, 112.7, 111.8, 110.3, 97.5, 67.7, 67.0, 63.1, 55.5, 26.3, 24.6, 22.0;
[0085] 1H NMR (400MHz, DMSO) δ (ppm): 8.01-8.03 (1H,q), 7.70-7.73 (1H,dd), 7.61-7.63 (1H,dd), 7.45-7.46 (1H,d), 7.30- 7.34...
Embodiment 3-6
[0087] According to the method described in Example 2, different organic solvents and oxidizing agents were selected to obtain the compound of formula I, and the results are shown in Table 1 below:
[0088] Table 1
[0089] Numbering Organic solvents oxidizing agent HPLC purity of compound of formula Ⅰ Example 3 N,N-Dimethylformamide hydrogen peroxide 97.1% Example 4 Acetonitrile tert-butyl hydroperoxide 98.3% Example 5 Acetonitrile m-chloroperoxybenzoic acid 98.6% Example 6 Acetonitrile peracetic acid 97.8%
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com