High-yield purification method for preparation of high-purity sildenafil freebases
A technology of sildenafil and purification method, which is applied in the purification field of removing new impurities from sildenafil free base, and can solve problems such as undetectable impurities and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
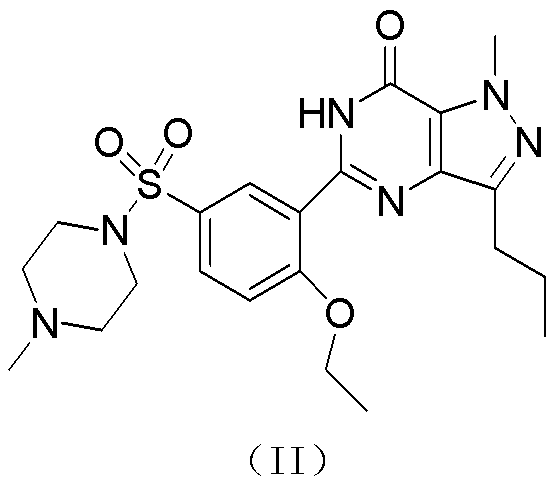
[0068] Example 1 Preparation of Sildenafil Free Base (II) Crude Product
[0069] Among them, 4-amino-1-methyl-3-n-propylpyrazole-5-carboxamide (VII) comes from Quzhou Yongchuang Chemical Technology Co., Ltd., and o-ethoxybenzoyl chloride (VI) comes from Zibo Yi New Chemical Co., Ltd., and other reagents were from Sinopharm Chemical Reagent Beijing Co., Ltd.
[0070] 1) Synthesis of 1-methyl-3-propyl-4-(2-ethoxybenzamido)pyrazole-5-carboxamide (V)
[0071] Add 500ml of dichloromethane into a 2L reaction flask, then add 100g of (VII), start stirring, then add 83g of triethylamine, then cool in a low-temperature bath to 0°C, add 110g of (VI) dropwise, and keep it warm at room temperature for 4 hours after dropping , and then add water to wash once, let stand for liquid separation, and directly divide the obtained dichloromethane layer into two equal parts, concentrate and evaporate to dryness.
[0072] Method A: Refined with 100ml ethyl acetate, filtered and dried to obtain 75g...
Embodiment 2
[0086] Example 2 Preparation of Sildenafil Free Base (II) Essence
[0087] Add 15ml of toluene and 2ml of methanol to a 100ml reaction flask, add 5g of crude sildenafil free base (II), stir well, heat up to 45~50°C, keep stirring for 2h, then cool to room temperature, filter, and dry to obtain Product (II).
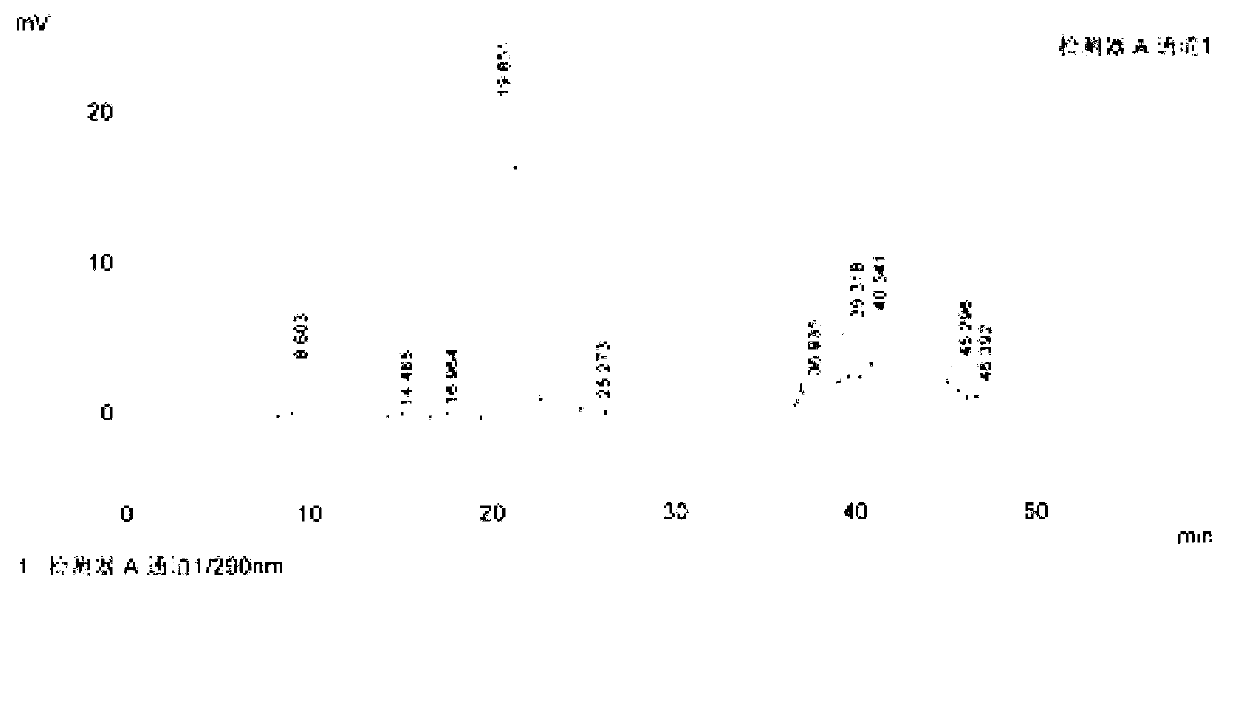
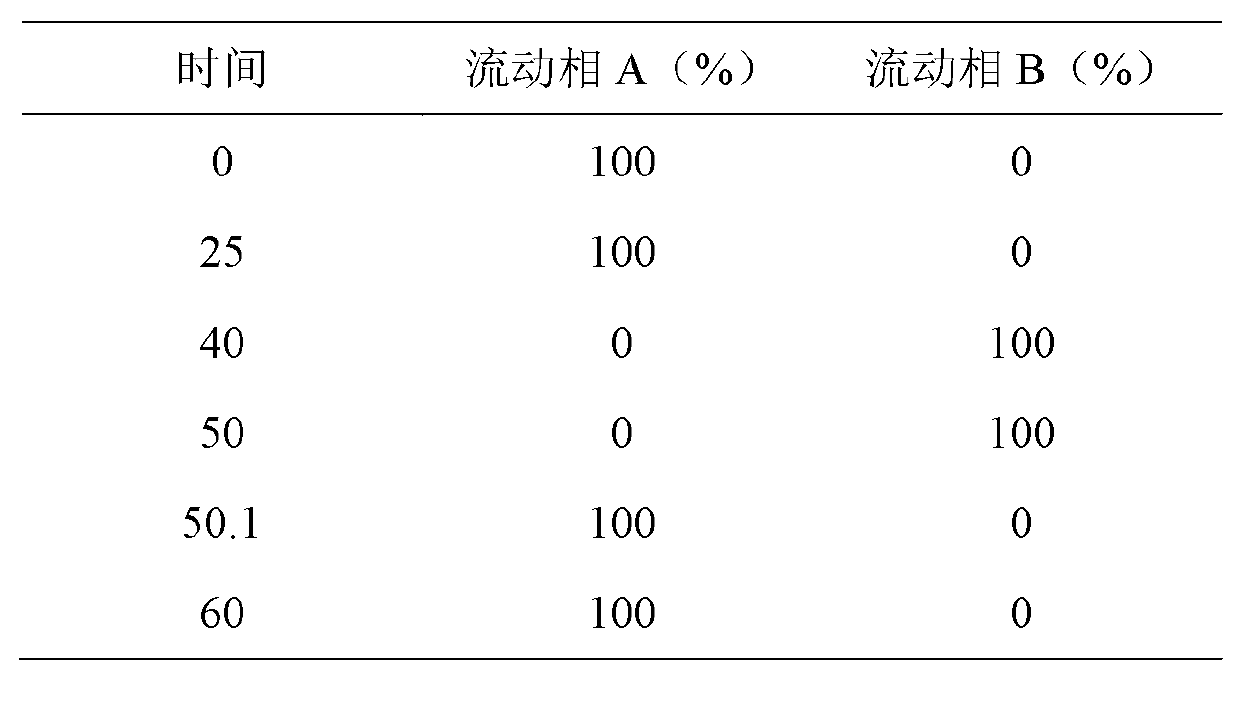
[0088] Method A: Yield 92.8%, wherein the content of impurity I08 is 0.058%
[0089] Method B: Yield 93.4%, wherein the content of impurity I08 is 0.028%
Embodiment 3
[0090] Example 3 Preparation of Sildenafil Free Base (II) Essence
[0091] Add 15ml of toluene and 4ml of methanol to a 100ml reaction flask, add 5g of crude sildenafil free base (II), stir well, heat up to 45~50°C, keep stirring for 2h, then cool to room temperature, filter, and dry to obtain Product (II).
[0092] Method A: Yield 90.6%, wherein the content of impurity I08 is 0.054%
[0093] Method B: Yield 91.2%, wherein the content of impurity I08 is 0.025%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com