Sulfobutyl-alpha-cyclodextrin and preparation method thereof
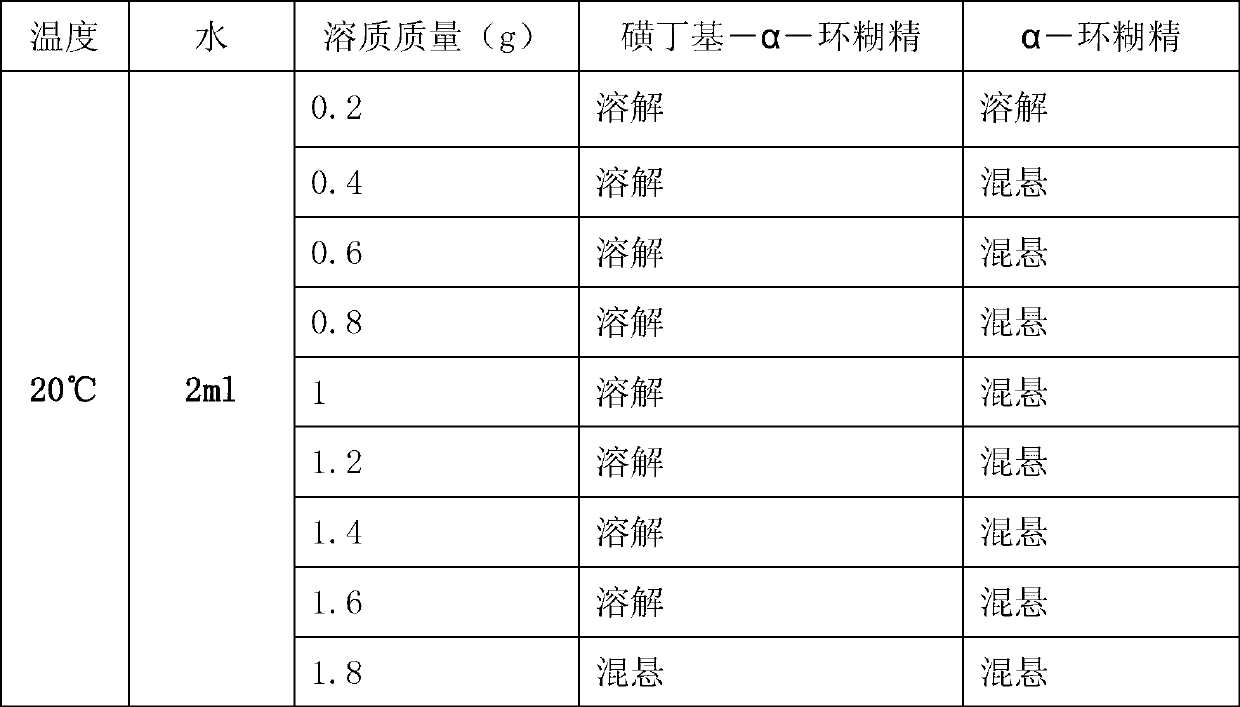
A technology of cyclodextrin and sulfobutyl, which is applied in the field of sulfobutyl-α-cyclodextrin and its preparation, can solve the problems of low solubility and application limitations of α-cyclodextrin, and achieve good water solubility and stability Strong, operable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, sulfobutyl-α-cyclodextrin and its preparation method:
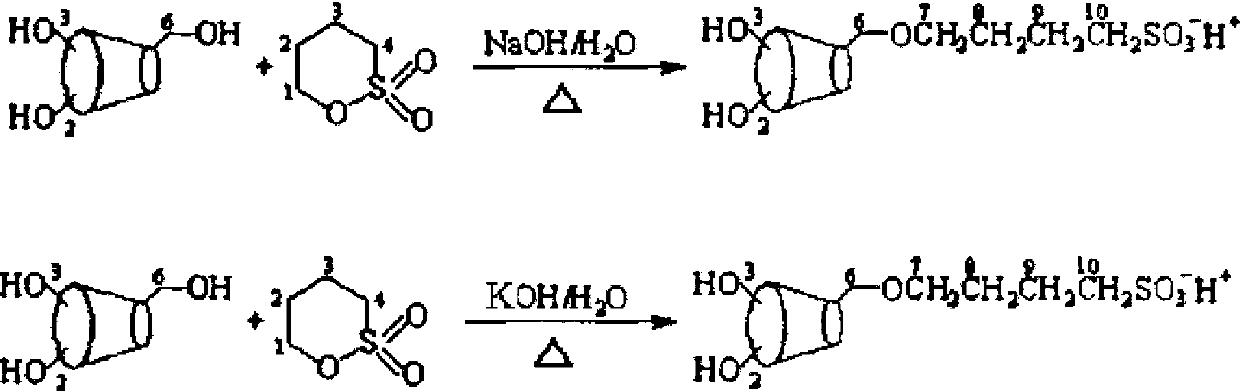
[0035] (1) First weigh 10g of α-cyclodextrin and mix it with 2g of sodium hydroxide, add 50ml of water, stir slowly until swelling, adjust the pH value to 14, and set aside;
[0036] (2) Weigh 2 g of 1,4-butane sultone, add 1,4-butane sultone dropwise to step (1) for reaction, then heat the reaction at 30°C for 3 hours to fully react until At the end of the reaction, the product is ready for use;
[0037] (3) Under stirring, wash the product obtained in step (2) with chloroform, adjust the pH value to neutral with 0.5mol / L hydrochloric acid, then evaporate the organic solvent, and freeze-dry at -40°C to obtain colorless crystals Sulfobutyl-α-cyclodextrin;
[0038] (4) The measured yield is greater than 60%;
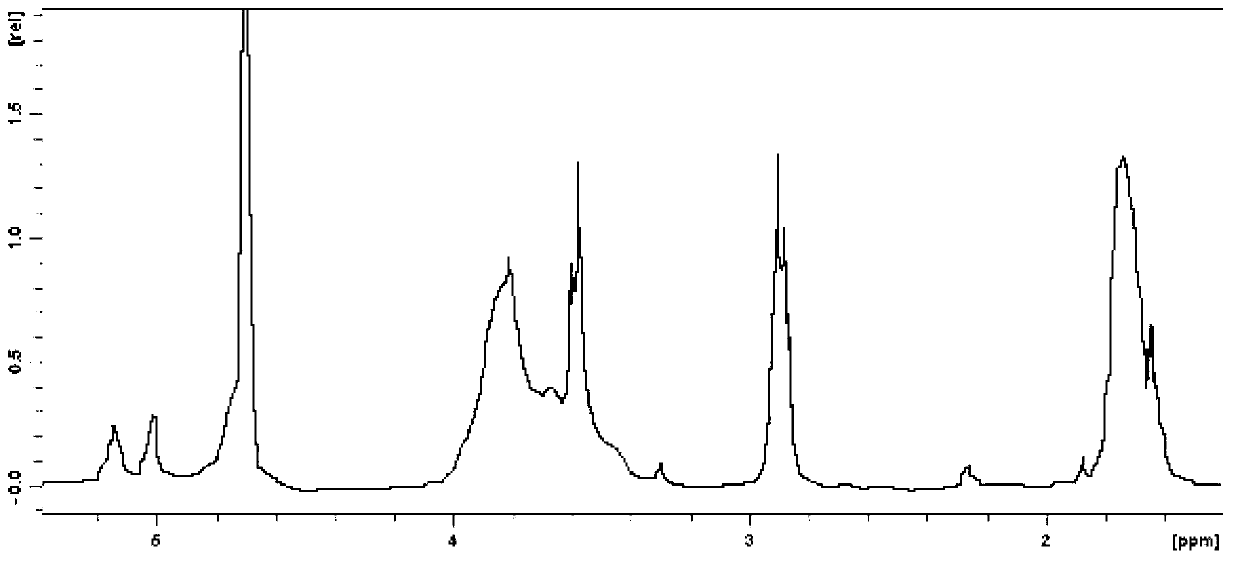
[0039] (5) Measure its molecular weight to be 1108.17, and its chemical formula is C 36+4n h 60+7n o 30 [SO 3 H] n , n=1.
Embodiment 2
[0040] Embodiment 2, sulfobutyl-alpha-cyclodextrin and its preparation method:
[0041] (1) First weigh 10g of α-cyclodextrin and mix it with 0.1g of sodium hydroxide, add 50ml of water, stir slowly until swelling, adjust the pH value to 10, and set aside;
[0042] (2) Weigh 5 g of 1,4-butane sultone, add 1,4-butane sultone dropwise to step (1) for reaction, then heat the reaction at 80°C for 3 hours to fully react until At the end of the reaction, the product is ready for use;
[0043] (3) Under stirring, wash the product obtained in step (2) with chloroform, adjust the pH value to neutral with 0.5mol / L hydrochloric acid, then evaporate the organic solvent, and freeze-dry at -40°C to obtain colorless crystals Sulfobutyl-α-cyclodextrin;
[0044] (4) The measured yield is greater than 60%;
[0045] (5) measure its molecular weight to be 1653, chemical formula is C 36+4n h 60+7n o 30 [SO 3 H] n , n=5.
Embodiment 3
[0046] Embodiment 3, sulfobutyl-α-cyclodextrin and its preparation method:
[0047] (1) First weigh 10g of α-cyclodextrin and mix it with 0.5g of sodium hydroxide, add 50ml of water, stir slowly until swelling, adjust the pH value to 12, and set aside;
[0048] (2) Weigh 20 g of 1,4-butane sultone, add 1,4-butane sultone dropwise to step (1) for reaction, then heat the reaction at 30°C for 18 hours to fully react until At the end of the reaction, the product is ready for use;
[0049] (3) Under stirring, wash the product obtained in step (2) with chloroform, adjust the pH value to neutral with 0.5mol / L hydrochloric acid, then evaporate the organic solvent, and freeze-dry at -40°C to obtain colorless crystals Sulfobutyl-α-cyclodextrin;
[0050] (4) The measured yield is greater than 60%;
[0051] (5) measure that its molecular weight is 1789, and its chemical formula is C 36+4n h 60+7n o 30 [SO 3 H] n , n=6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com