Method for formulating large diameter synthetic membrane vesicles
A technology of atomizing nozzles and contact chambers, which is applied in the field of pharmaceutical sciences and can solve the problems of manufacturing space, cost and time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
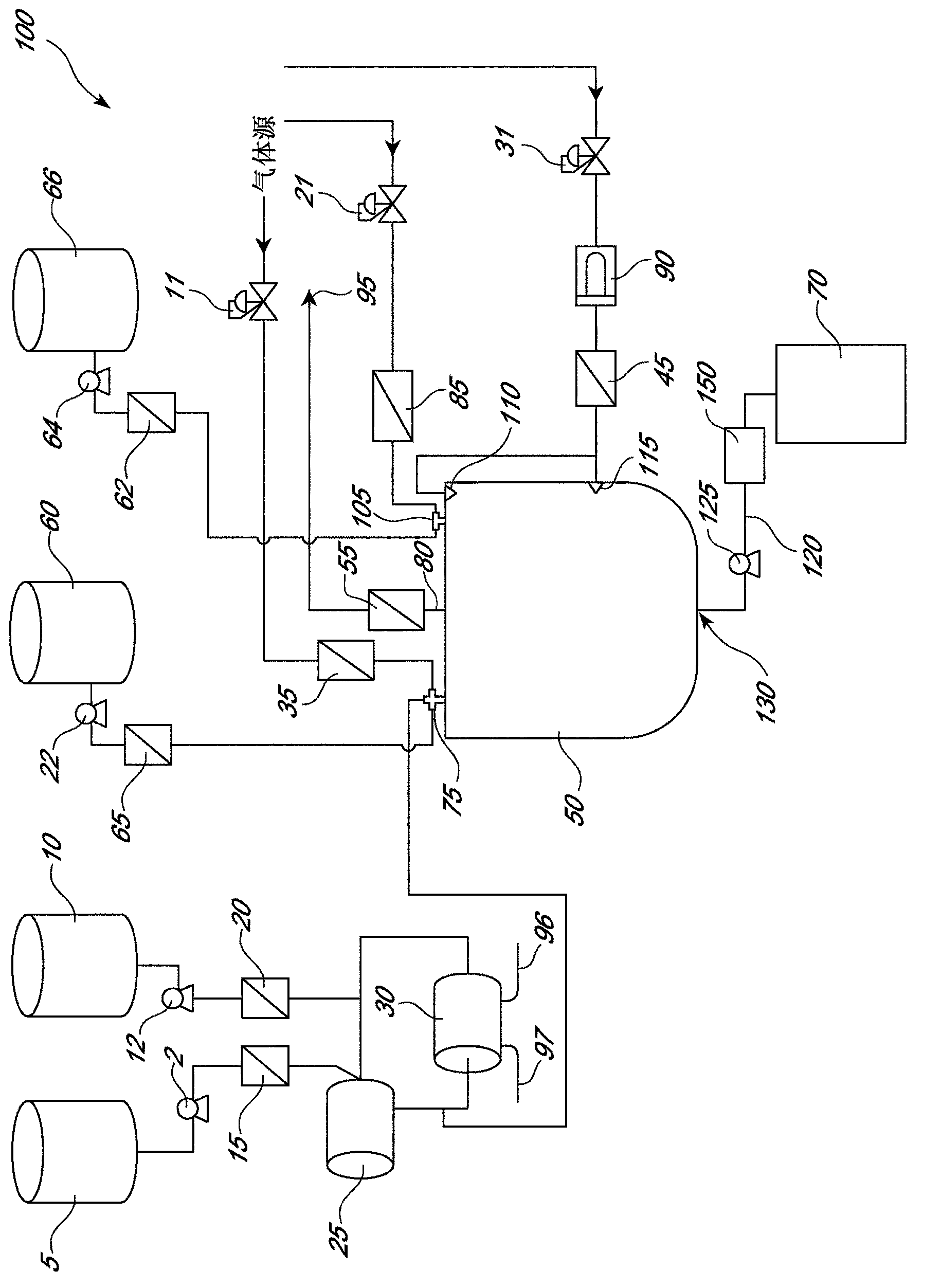
[0337] The following is one example of processing parameters and procedures using the apparatus shown in these figures. Applied to an atomizing nozzle as part of the process for forming multivesicular liposomes ( Figure 1A and Figure 1B , component 75; Figure 3A , component 310; Figure 7 , Part 7510) of the three fluids per liter have the following composition.
[0338] first fluid ( Figure 3A to Figure 3L , component 3115; Figure 5 , component 5115; Figure 7 , component 7115) is a first liquid composed of a first component having two components: an organic phase and a first aqueous phase, which are emulsified in equal volumes. The organic phase consisted of 1,2-dierucoyl-sn-glycero-3-phosphocholine (17.78 g), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (1.056 g), cholesterol (10.34 g), tricaprylin (4.32g), water (0.70g) and dichloromethane (enough to make 1L total volume of the organic phase). The first aqueous phase consisted of 0.2 molar (200 mM) phosphoric ac...
example 2
[0354] Preparation of the first component
[0355] Fill with dichloromethane connected to a high shear mixer ( Figure 1A and Figure 1B , component 25; figure 2 , part 2130) (Ross model HSM-703XS-20Sanitary Inline High Shear Mixer fitted with a 3" diameter X-5 series rotor / stator with #3 gap ring for operation to 14,400 rpm (11,300 ft / min, tip speed)) The recirculation loop on the top, thus ensuring that all air is removed from the high shear mixer. The jacket of the heat exchanger is supplied with a 5 °C coolant (water + 50% ethylene glycol) ( Figure 1A and Figure 1B , component 30; figure 2, part 2170). The mixer seal lubricant tank filled with water was also cooled with 5°C coolant (water + 50% ethylene glycol). Start the high shear mixer at a setting of 25Hz (6,000rpm), approximately 30Hz (7,200rpm), or 35Hz (8,400rpm).
[0356] After charging the high-shear mixer with dichloromethane, start the organic phase and first aqueous phase peristaltic pumps simultaneous...
example 3
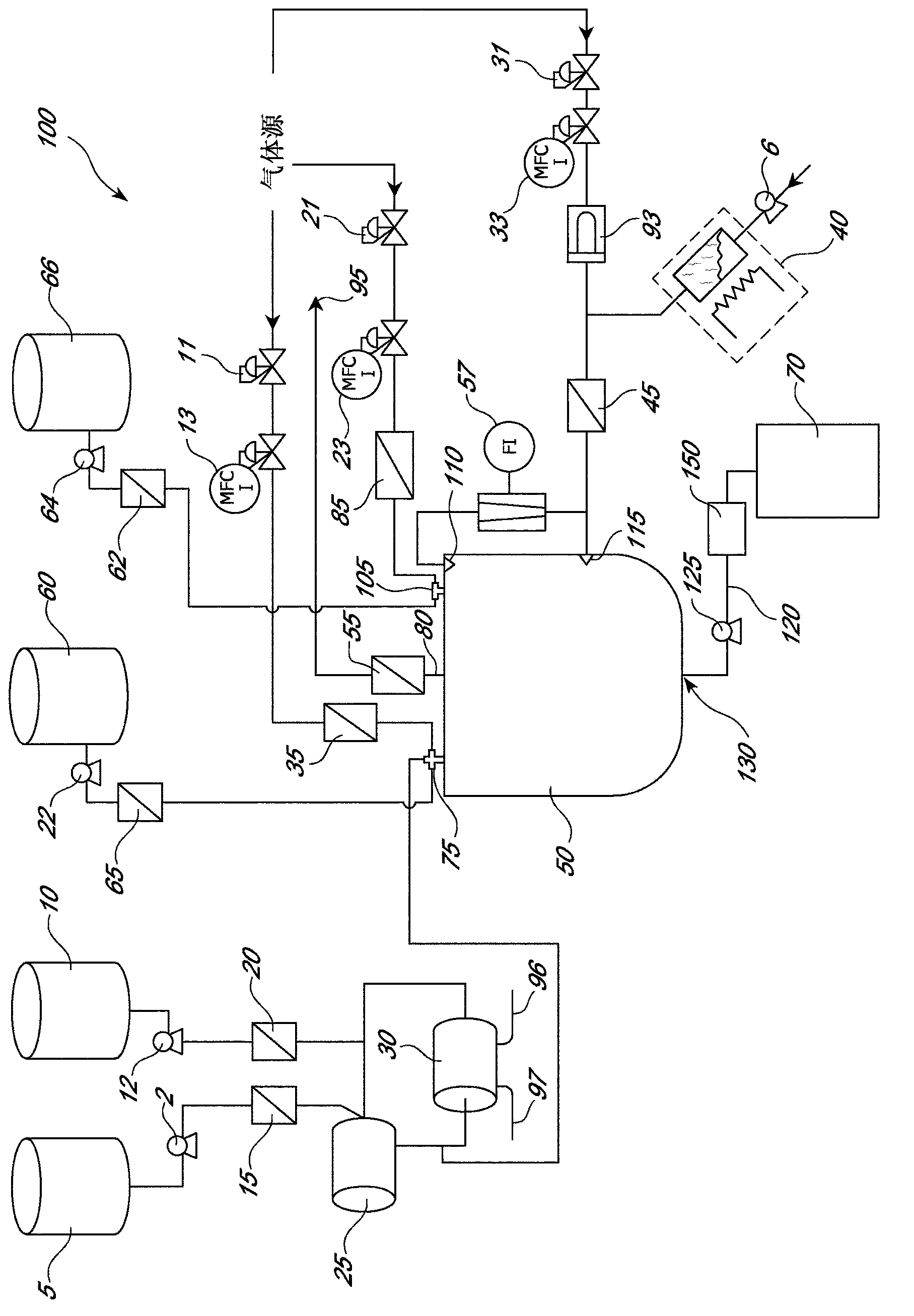
[0388] Heat treatment of MVL suspension
[0389] Will Figure 1B The system is connected with an electric heater and a tube-in shell heat exchanger (such as Figure 1A described, component 90) combined with a supply of humidified rotating gas (N 2 )use together. The system was equilibrated for 10 minutes, and the discharge exiting the solvent removal vessel 50 was collected ( Figure 1B , part 130) of a 1,000 ml sample of the MVL suspension. Divide the MVL sample into two samples of 500ml each. The first 500ml MVL sample was heat treated as follows. Heat treatment was performed by rapidly adding 750 ml of 100°C dextrose solution to the first sample, raising the temperature of the mixture to approximately 63°C. After 30 seconds, 1,750 ml of +5°C brine was added quickly, thereby reducing the temperature of the mixture to near room temperature (35°C or lower). Now, the sample volume is 3,000ml. A second 500 ml sample of multivesicular liposomes was not heat-treated. The s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com