Separation method of triethylene diamine and ethanolamine azeotrope
A triethylenediamine and separation method technology, which is applied in the field of separation and purification of fine chemicals, can solve the problems of high energy consumption and large amount of entrainer used, and achieve the goal of reducing energy consumption, reducing energy consumption, and reducing the amount of use Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
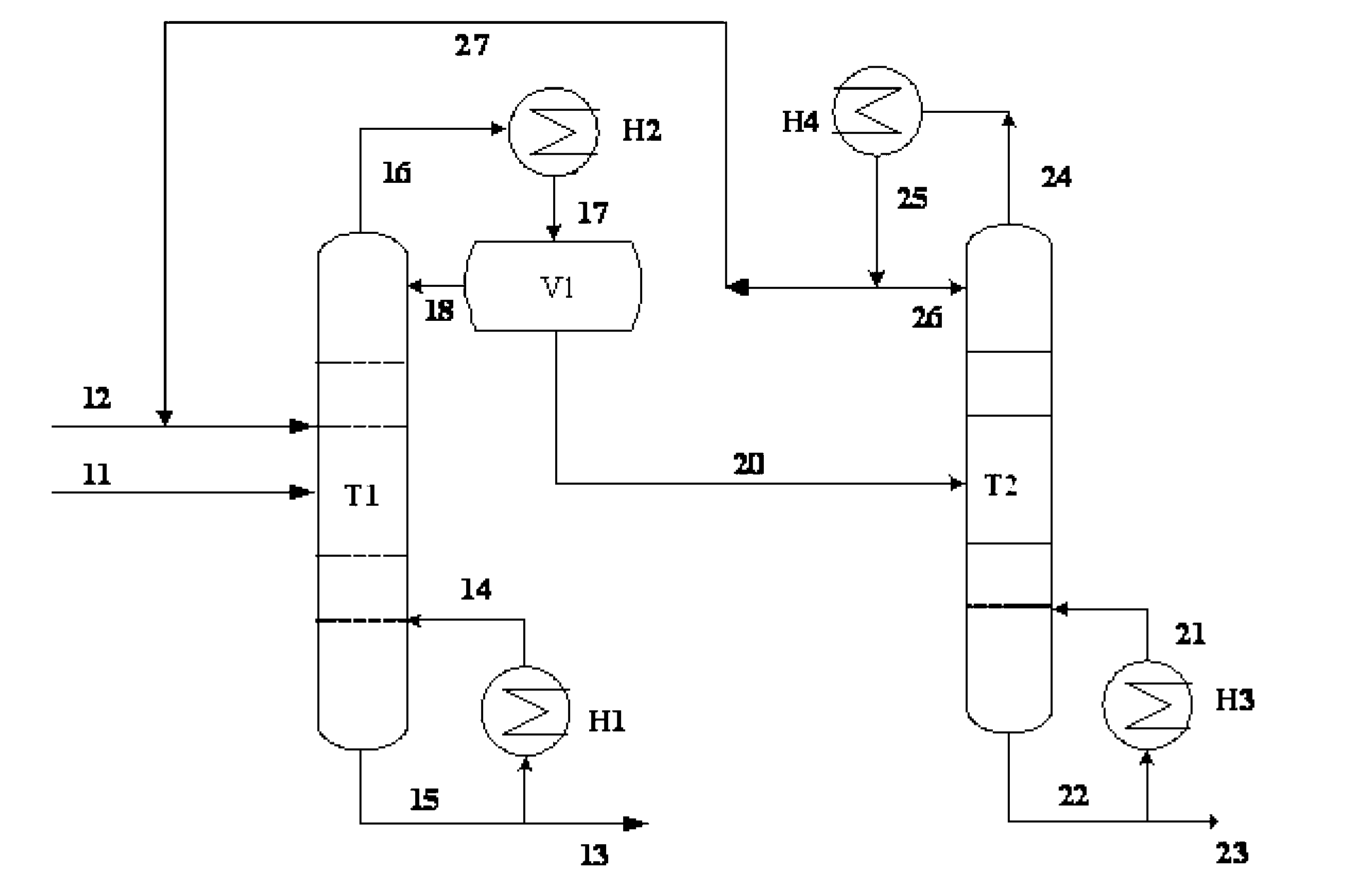
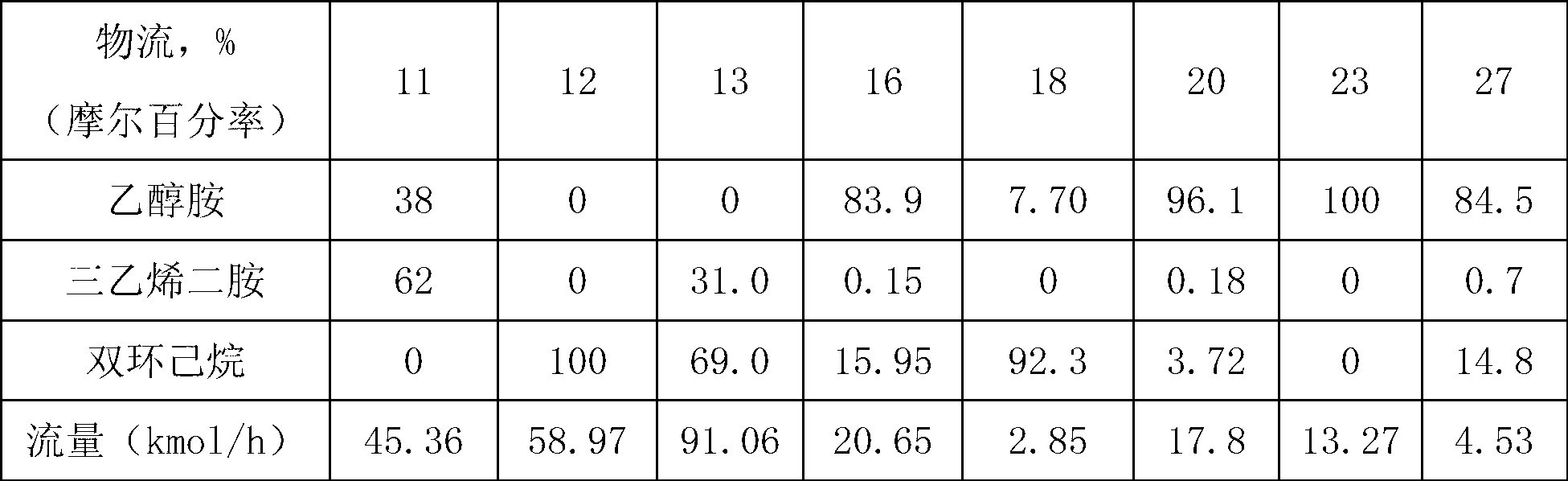
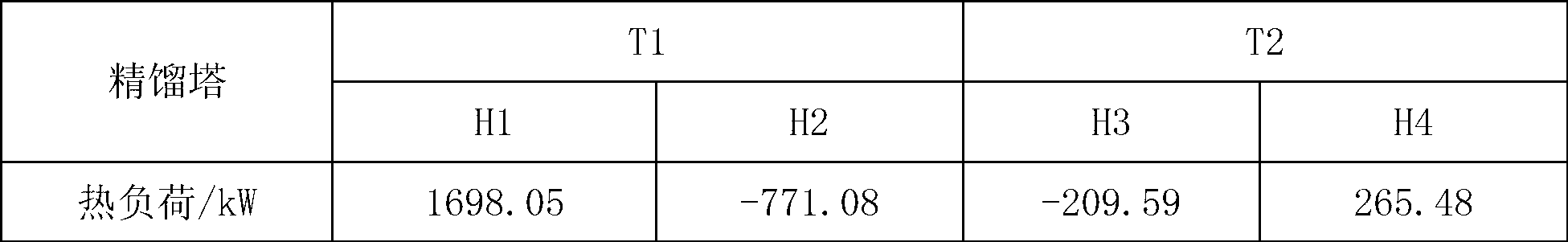
[0028] Example 1: The azeotrope and entrainer of triethylenediamine and ethanolamine enter the azeotropic rectification tower, the overhead fraction is the azeotrope of the entrainer and ethanolamine, and the bottom fraction is triethylenediamine and the entrainer mixture, the mixture is cooled and crystallized to precipitate solids, and the solid product obtained after filtration is triethylenediamine. The purity of the product is 99.8%. The entrainer is dicyclohexane, and ethanolamine and dicyclohexane The molar ratio of the azeotropic distillation column is 1:1.25, the operating pressure of the azeotropic distillation column is 20kPa, the temperature at the top of the column is 122.4°C, the temperature at the bottom of the column is 145.8°C, and the reflux ratio is 1.5; the top fraction of the azeotropic distillation column T1 enters the phase separation The phase separator V1 is used for phase separation, the upper layer is the entrainer, which is circulated to the azeotrop...
Embodiment 2
[0033] Example 2: The azeotrope and entrainer of triethylenediamine and ethanolamine enter the azeotropic rectification tower T1. agent mixture, the solid product obtained after cooling, crystallization and filtration of the mixture is triethylenediamine with a purity of 99.8%. The entrainer is decahydronaphthalene, ethanolamine and decalin in the azeotrope The molar ratio of the azeotropic distillation column is 1:2.25, the operating pressure of the azeotropic distillation column is 20kPa, the temperature at the top of the column is 113.5°C, the temperature at the bottom of the column is 126.1°C, and the reflux ratio is 1.5; the top fraction of the azeotropic distillation column T1 enters the phase separation The phase separator V1 is used for phase separation, the upper layer is an azeotropic agent, which is circulated to the azeotropic distillation tower, and the lower layer is the crude ethanolamine, which enters the ethanolamine refining tower T2, wherein the operating pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com