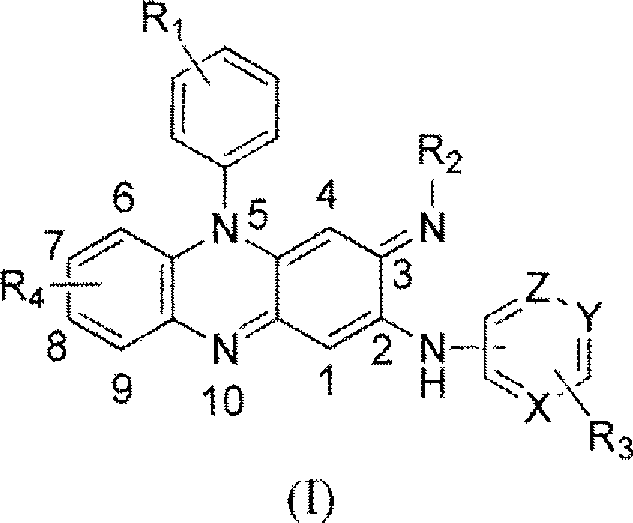
Riminophenazines with 2-(heteroaryl)amino substituents and their anti-microbial activity
A technology of substituents and cycloalkylamino groups, applied in the field of treatment of Mycobacterium tuberculosis and other microbial infections, can solve the problems of skin discoloration, high fat tissue distribution, long half-life and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 1
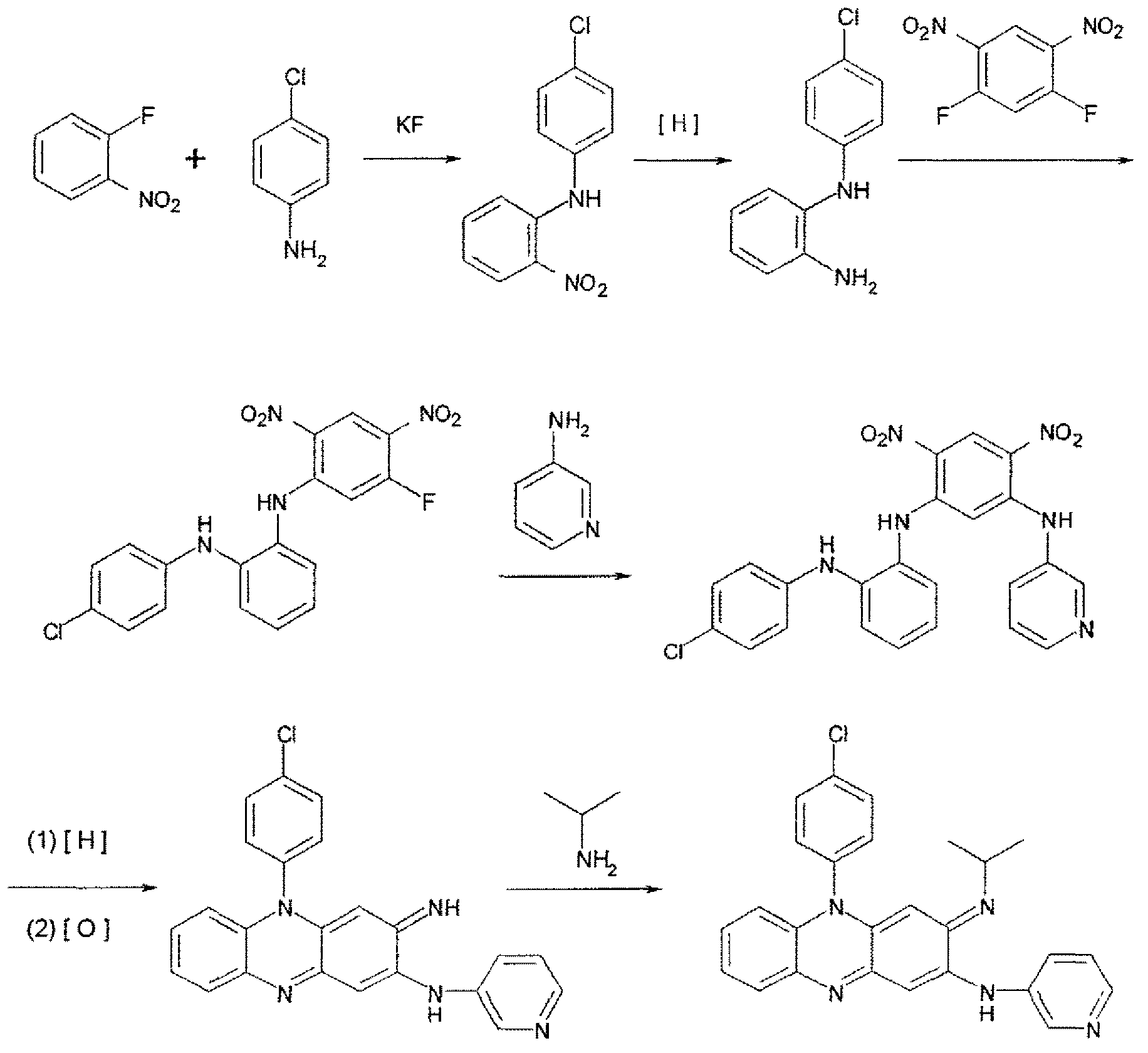
[0079] Embodiment 1. General synthetic method
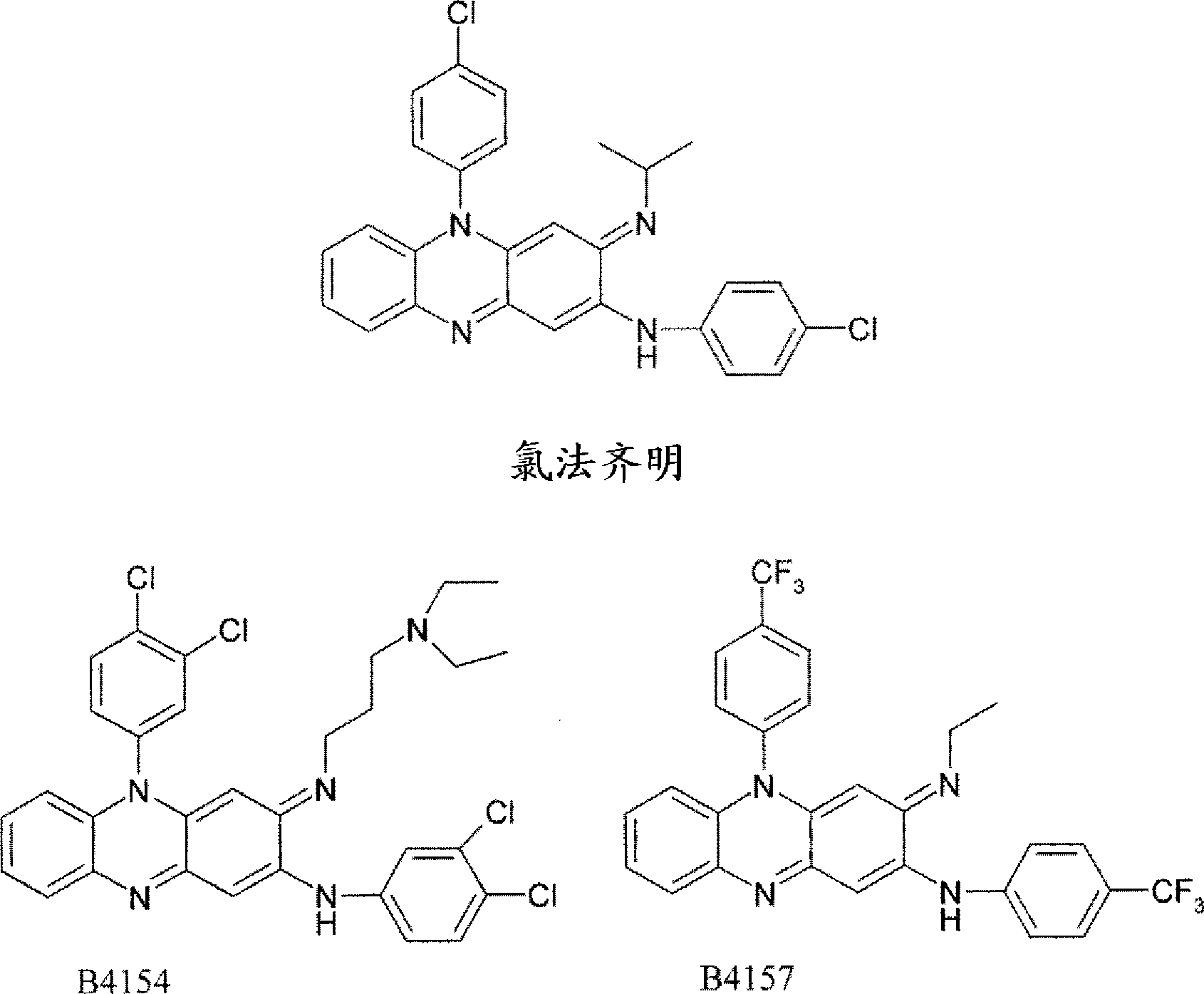
[0080] The following provides the preparation of 5-(4-chlorophenyl)-3-(1-methylethyl)imino-2-(3-pyridyl)amino-3,5-dihydrophenazine (TBI-416) Or a general method for the clofazine compounds of the invention.
[0081] Step A: 2-(4-Chloroanilino)-nitrobenzene
[0082] A mixture of 2-fluoro-nitrobenzene (33.7 g), 4-chloroaniline (61.0 g) and anhydrous potassium fluoride (13.9 g) was stirred at 180° C. for 10 hours. After cooling to room temperature, 3M HCl was added and the mixture was stirred at 100°C for 30 minutes. It was then cooled to room temperature, filtered by suction and washed with water to give a brown solid. Dissolve the solid in CH 2 Cl 2 medium, and filtered through a thin pad of silica gel, with CH 2 Cl 2 washing. The filtrate was concentrated to dryness, and the residue was recrystallized from 95% ethanol to give 57.0 g of an orange solid.
[0083] Step B: 2-(4-Chloroanilino)-aniline
[0084] To the CH of 2...
Embodiment 2
[0124] Example 2 In Vitro Determination of Antimicrobial Susceptibility
[0125] Antimicrobial susceptibility testing was performed in 96-well plates. Initial drug dilutions (6.4 mg / ml) were prepared in dimethylsulfoxide followed by two-fold dilutions in 0.1 ml of 7H9 in microplates. The final drug concentration was about 0.008 μg / ml. Each concentration of test compound was added to two wells. Control wells consisted of bacteria and positive drug (clofazimine). Plates were incubated at 37°C. The final bacterial titer was for H 37 Rv 1 x 10 6 CFU / ml. From day 7 of incubation, add 20 μl of 10× Alamar blue solution and 12.5 μl of 20% Tween 80 to each well and re-incubate the plate at 37 °C. Wells were observed at 24 hours and the color of all wells was recorded. The visual MIC (visual MIC) was defined as the lowest amount of drug that prevented the color from blue to pink. Fluorescence was measured in a microplate fluorometer in bottom reading mode with excitation at 530...
Embodiment 3
[0126] Example 3 in vivo test
[0127] Use 0.2ml containing 1×10 5 CFU of H 37 The dose of RV was used to infect male BALB / c mice (18-20 g) intravenously. One day after infection, four mice were sacrificed and the number of CFU in spleen and lung was determined. Organs were removed and homogenized in Middlebrook 7H9 medium. To calculate CFU, appropriate dilutions of the homogenate were plated onto Middlebrook 7H10 agar and colony counts were performed after incubation at 37°C for 3 to 4 weeks. The remaining mice were assigned to untreated or multiple drug-treated groups (six mice per group). The dose of each test compound was 20 mg / kg. The positive control group was treated with isoniazid and clofazimine. Test compounds and clofazimine were administered 5 times per week by gavage. CMC was administered to untreated mice by gavage. 30 days after infection, untreated and treated mice were sacrificed to determine the CFU count in lung tissue. Significance of differences i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com