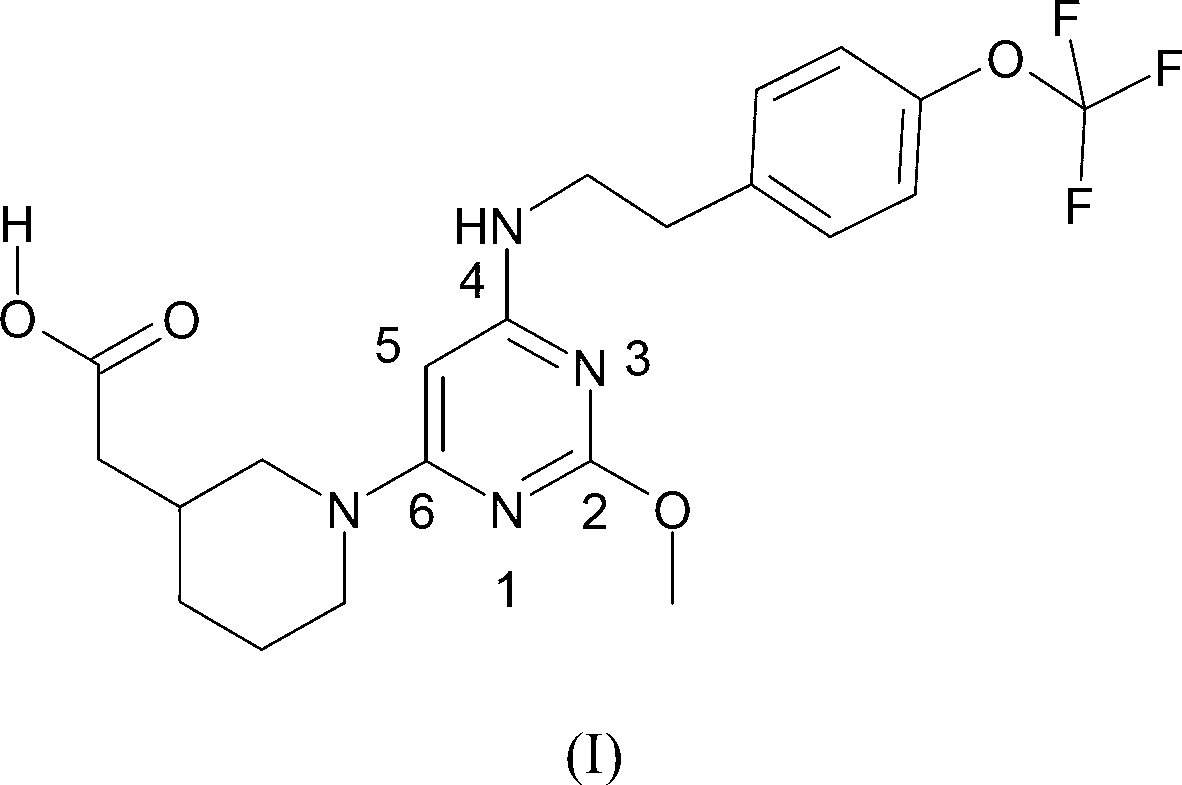
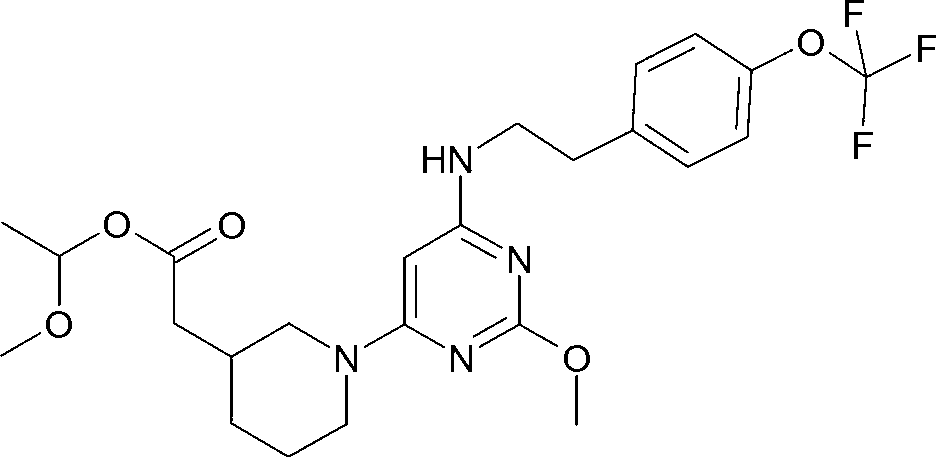
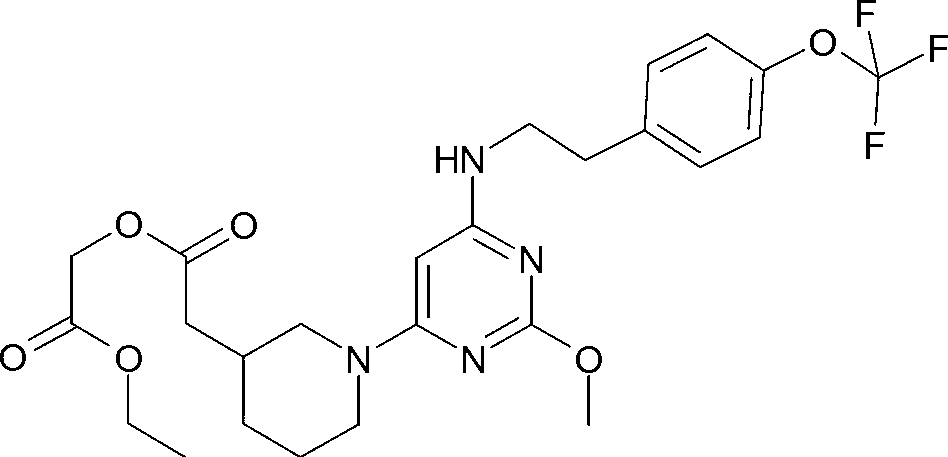
A substituted pyrimidine as a prostaglandin d2 receptor antagonist
A technology of isomers and ester prodrugs, applied in metabolic diseases, medical preparations containing active ingredients, allergic diseases, etc., can solve problems such as the suspension of niacin treatment and the impact of patients' compliance with doctor's orders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0126] Reaction Scheme of Compound 1
[0127]
[0128] step 1
[0129] 2-(4-Trifluoromethoxy-phenyl)-ethylamine hydrochloride. (3)
[0130]
[0131] To a 500 mL hydrogenation reactor was added a solution of (4-trifluoromethoxy-phenyl)-acetonitrile (2) (25.0 g, 124.28 mmol), hydrochloric acid (12N, 25.89 mL, 310.70 mmol) in 200 mL of methanol and palladium / carbon (5wt%, 13.00g). The reactor was placed in a Parr-shaker and hydrogenated overnight (17 hours) at room temperature under 55 PSI hydrogen pressure. The catalyst was removed by filtration through a pad of celite, and the filtrate was concentrated under reduced pressure. The solid residue was dissolved in ethyl acetate / dichloromethane (300 mL, 1:1 v / v) and diluted slowly with 200 mL of heptane with vigorous stirring. The precipitated amine salt was collected by filtration to give the title compound (3) (25.50 g, 85%). LC / MS: Rt=1.96 min, MS m / z=206.
[0132] step 2
[0133] (6-Chloro-2-methoxy-pyrim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com