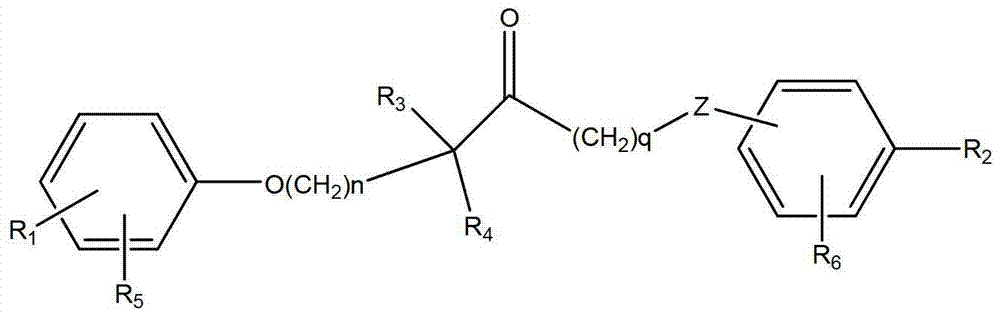
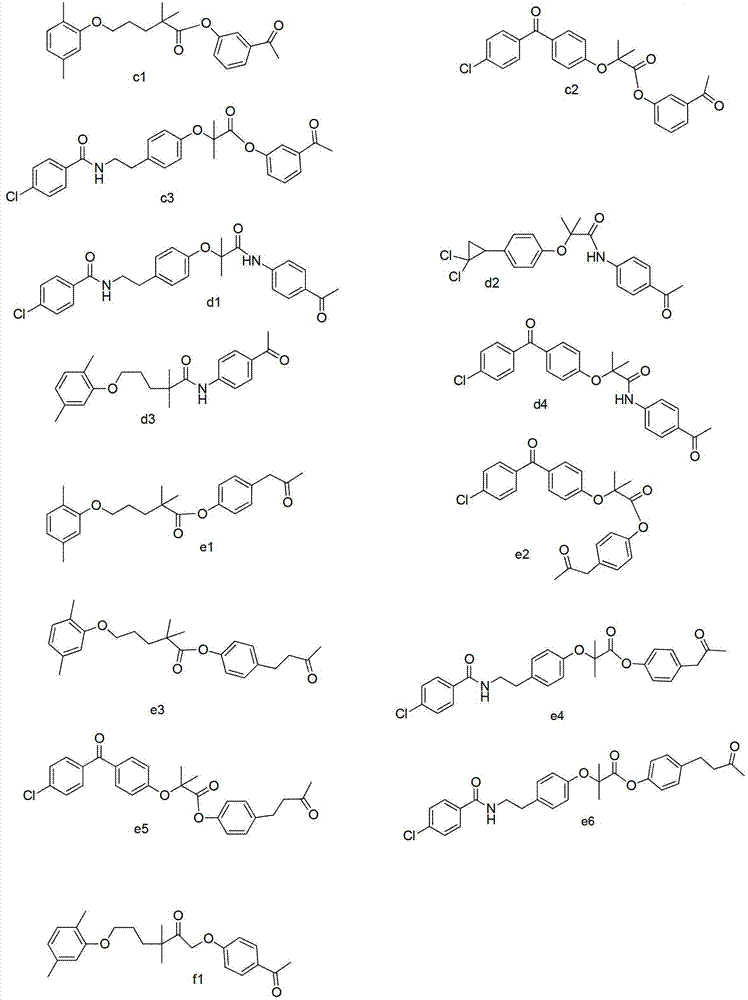
Carboxylic acid derivatives compound and preparation method and application thereof
A compound and alkyl technology, applied in the field of carboxylic acid derivatives and their preparation and application, can solve the side effects of rhabdomyolysis syndrome and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
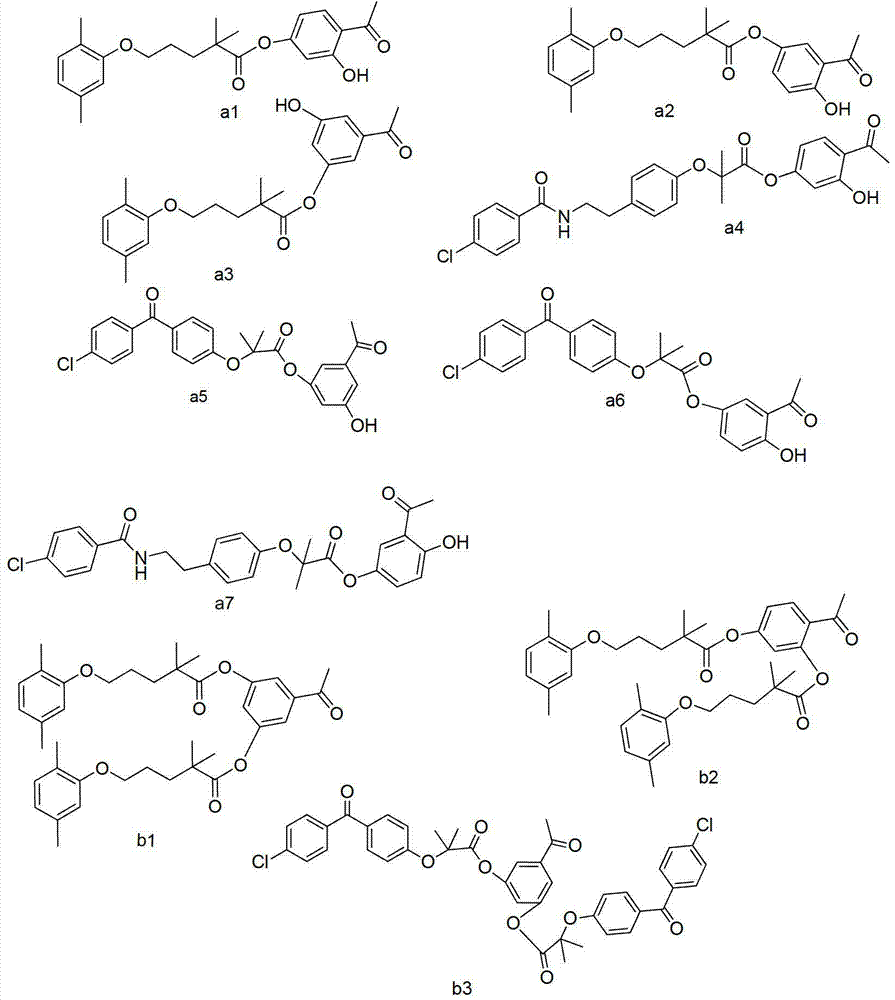
[0087] Embodiment 1: the preparation of compound a1
[0088] Gemfibrozil 1g, dicyclohexylcarbodiimide 1.07g, 2,4‐dihydroxyacetophenone 0.608g, dichloromethane 60ml in a 100ml one-mouth bottle, react at room temperature for 5 hours, evaporate to dryness, ethanol Recrystallization gave 1.19 g of compound a1. MS (ESI) 385 (M+H).
Embodiment 2
[0089] Embodiment 2: the preparation of compound a2
[0090]Gemfibrozil 1g, dicyclohexylcarbodiimide 1.07g, 2,5-dihydroxypropiophenone 0.608g, dichloromethane 60ml in a 100ml one-mouth bottle, react at room temperature for 5 hours, evaporate the solvent to dryness, and ethanol weight Crystallization gave 1.21 g of compound a2. MS (ESI): 385 (M+H + ).
Embodiment 3
[0091] Embodiment 3: the preparation of compound a3
[0092] Gemfibrozil 1g, dicyclohexylcarbodiimide 1.07g, 3,5-dihydroxyacetophenone 0.608g, dichloromethane 60ml were placed in a 100ml one-mouth bottle, reacted at room temperature for 5 hours, evaporated to dryness, Recrystallization from ethanol gave 1.18 g of compound a3. MS (ESI): 385 (M+H + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com