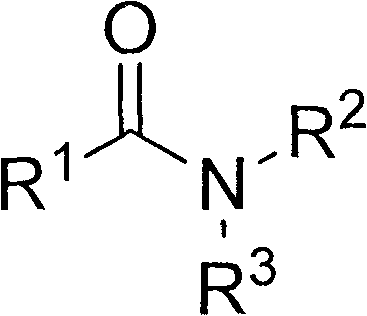
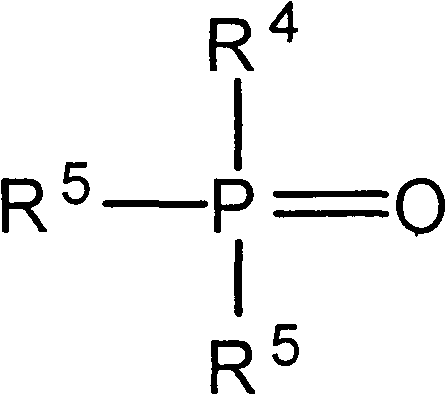
Method for extracting lithium salts in lithium-containing brine
A technology for extracting lithium and brine, applied in chemical instruments and methods, lithium compounds, solvent extraction of liquid solutions, etc., can solve the problems of strong corrosion of equipment and difficult industrialization, and achieve the effects of abundant sources, reduced dosage and optimized performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] In the extractant, tributyl phosphate is selected as the neutral phosphorus-oxygen compound, and the compound of the following structural formula is selected as the amide compound:
[0048]
[0049] In a separatory funnel, add 1 part of certain salt lake lithium-containing brine (Mg / Li=500 molar ratio) as shown in Table 1, add a certain amount of FeCl 3 (concentration in brine up to 0.05mol / L) as a co-extraction agent, shake to dissolve. Add 1 part of extractant (compared to O / A=1), the content of two extractants of amides and tributyl phosphate in the organic phase is 80% (V%), two extracts of amides and tributyl phosphate The relative volume percentage ratio (V / V) of the reagents is 20:1, and the extraction is carried out by shaking for less than 10 minutes. After the shaking is over, rest and stratify. Determination of Li in the equilibrium aqueous phase and organic phase, respectively + 、Na + 、K + and Mg 2+ , thus calculating the extraction rate of Li to be...
Embodiment 2
[0051] In the extractant, tributyl phosphate is selected as the neutral phosphorus-oxygen compound, and the compound of the following structural formula is selected as the amide compound:
[0052]
[0053] In a separatory funnel, add 1 part of certain salt lake lithium-containing brine (Mg / Li=140 molar ratio) as shown in Table 1, add a certain amount of FeCl 3 (concentration in brine up to 1.0mol / L) as a co-extraction agent, shake to dissolve it. Add 5 parts of extractant (compared to O / A=5), the content of amides and tributyl phosphate in the organic phase is 60% (V%), two extractions of amides and tributyl phosphate The relative volume percentage ratio (V / V) of the reagents is 10:1, and the extraction is carried out by shaking for 10 minutes. After the shaking is over, rest and stratify. Determination of Li in the equilibrium aqueous phase and organic phase, respectively + 、Na + 、K + and Mg 2+ Concentration, the extraction rate of Li calculated from this is 87.60%, ...
Embodiment 3
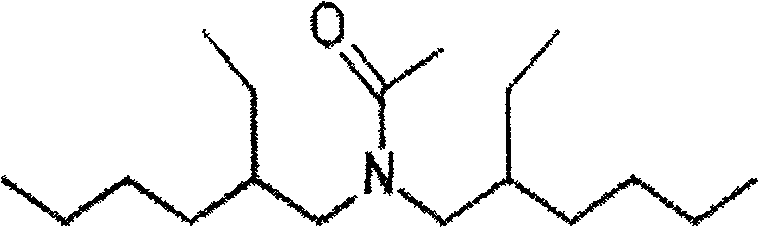
[0055] In the extractant, tributyl phosphate is selected as the neutral phosphorus oxygen compound, and N, N-di(2-ethylhexyl) acetamide is selected as the amide compound, and its structural formula is as follows:
[0056]
[0057] In a separating funnel, add 1 part of certain salt lake lithium-containing brine (Mg / Li=35 molar ratio) as shown in Table 1, add a certain amount of FeCl 3 (concentration in brine up to 3.5mol / L) as a co-extraction agent, shake to dissolve. Add 10 parts of extractant (compared to O / A=10), the content of amide compounds and tributyl phosphate in the organic phase is 30% (V%), two extractions of amides and tributyl phosphate The relative volume percentage ratio (V / V) of the reagents was 1:20, and the extraction was carried out by shaking for 10 minutes. After the shaking is over, rest and stratify. Determination of Li in the equilibrium aqueous phase and organic phase, respectively + 、Na + 、K + and Mg 2+ Concentration, the extraction rate of L...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com