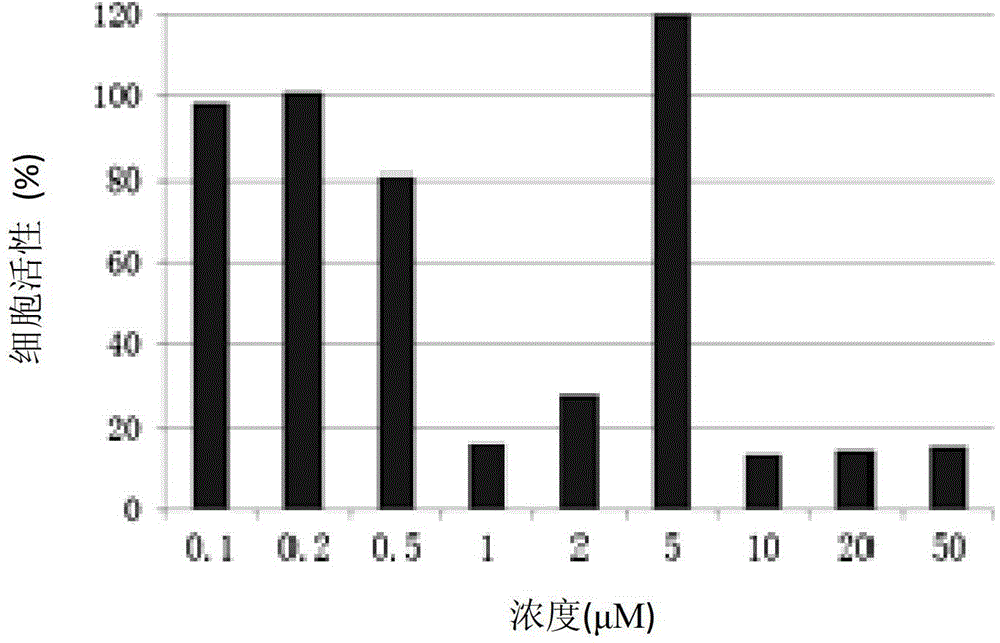
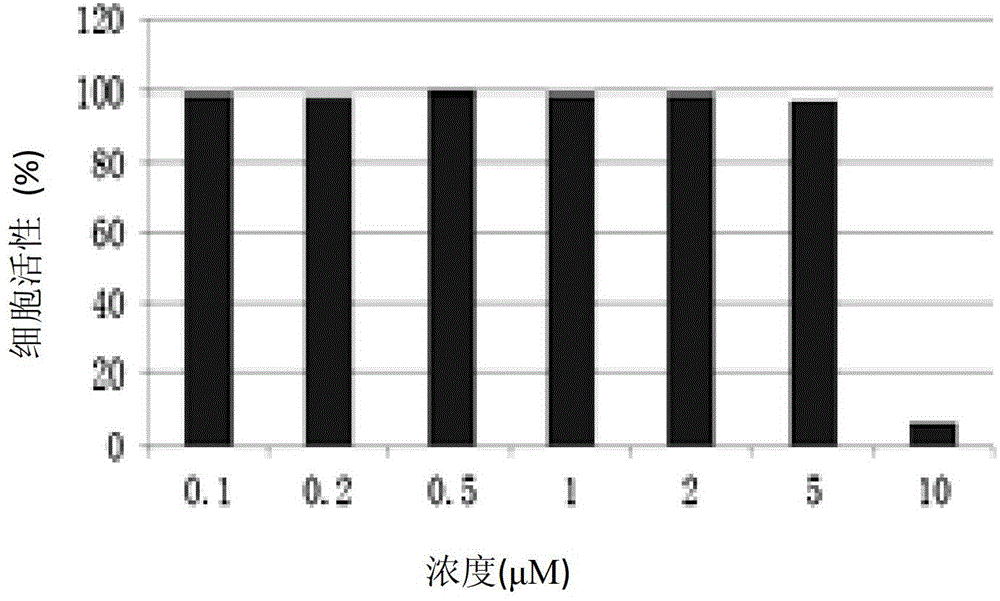
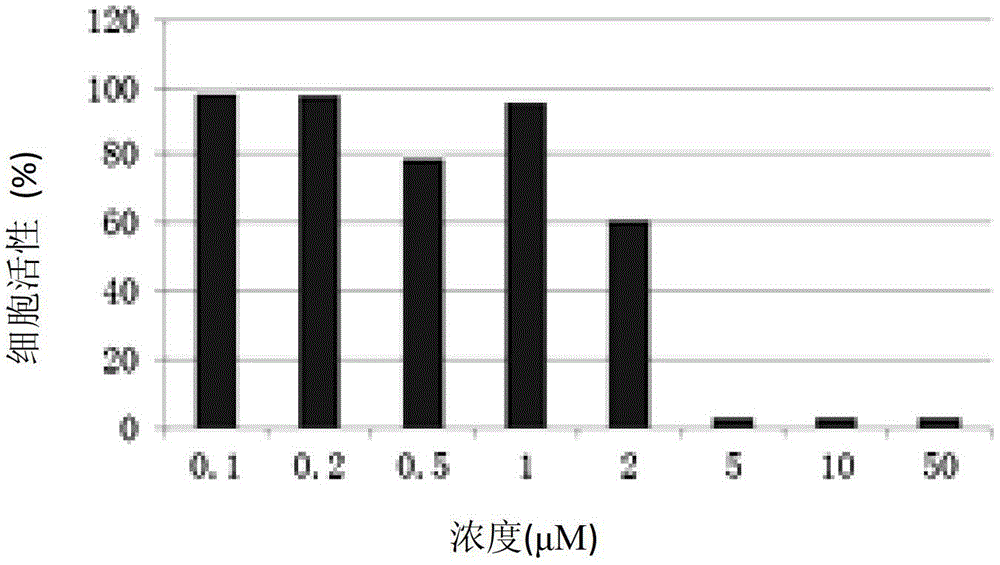
Pyrimidine compound with effect of adhesion kinase inhibition and preparation method and application thereof
A compound and pyrimidine technology, applied in the field of pyrimidine derivatives, can solve the problems of difficulty in providing diagnostic information, false positives, poor specificity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] The synthesis of compound 1, the synthetic route is as follows:
[0077]
[0078] Specific steps include:
[0079] 1.5g (10mmol) 2-chloro-7H-pyrrole[2,3-d]pyrimidine, 3g anhydrous potassium phosphate, a small amount of CuI and trans-1,2-cyclohexanediamine and 10ml dioxane were added to In the there-necked flask, then slowly add 3.2g (12mmol) 3-bromobenzophenone, at 110°C, N 2 Stirring and reflux under protection for 5h. The reaction was detected with a thin-layer chromatographic plate, the reaction was completed, the solid was filtered, and the solid was washed 3 times with a small amount of dioxane, and then saturated NaHCO was added. 3 Solution, the organic phase was washed with 3×10mL saturated brine, dried with anhydrous MgSO4, filtered, spin-dried dioxane, and passed through a silica gel column with a developer ethyl acetate:petroleum ether=1:10-1:2, and finally A pale white solid A was obtained with a yield of 75.5%. Add 300mg of compound A, 600mg of p-brom...
Embodiment 2
[0081] The synthesis of compound 2, the synthetic route is as follows:
[0082]
[0083] Concrete synthetic steps include:
[0084] Add 3.5g of 2,5-dibromobenzoic acid and 20ml of methanol into a 100ml single-necked bottle, stir at room temperature and slowly add 10ml of concentrated sulfuric acid, stir for 10 minutes after adding the concentrated sulfuric acid and start heating to reflux, react for 8 hours, and detect the reaction with a thin-layer chromatography plate. After the reaction, excess methanol was spun to dry, 50ml of water was added, and filtered to obtain a white solid with a yield greater than 85%. 1.5g (10mmol) 2-chloro-7H-pyrrole[2,3-d]pyrimidine, 3g anhydrous potassium phosphate, a small amount of CuI and trans-1,2-cyclohexanediamine and 10ml dioxane were added to In the three-necked flask, add 3.0 g of methyl 2,5-dibromobenzoate, and stir and reflux at 110° C. for 6 h. The reaction was detected with a thin-layer chromatographic plate, the reaction was ...
Embodiment 3
[0086] The synthesis of compound 3, the synthetic route is as follows:
[0087]
[0088] Concrete synthetic steps include:
[0089] 1.5g (10mmol) 2-chloro-7H-pyrrole[2,3-d]pyrimidine, 3g anhydrous potassium phosphate, a small amount of CuI and trans-1,2-cyclohexanediamine and 10ml dioxane were added to Then slowly add 4.5g of 2,5-dibromopyridine into the three-neck flask, and stir and reflux at 110°C for 5h. The reaction was detected with a thin-layer chromatographic plate, the reaction was completed, the solid was filtered, and the solid was washed 3 times with a small amount of dioxane, and then saturated NaHCO was added. 3 solution, the organic phase was washed with 3×10mL saturated brine, dried with anhydrous MgSO4, filtered, spin-dried dioxane, and passed through a silica gel column with a developer ethyl acetate:petroleum ether=1:10-1:2, and finally 950mg of pale white solid was obtained. Add 300mg of compound C, 600mg of anisidine, 3ml of concentrated hydrochloric...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com