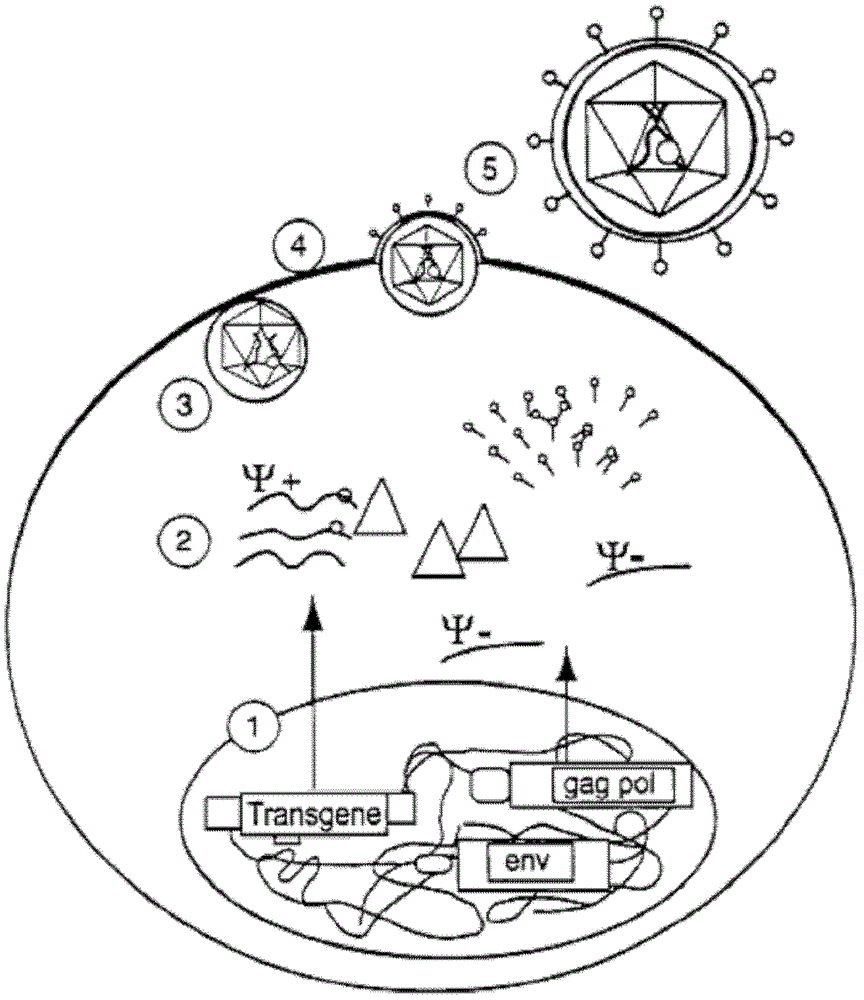
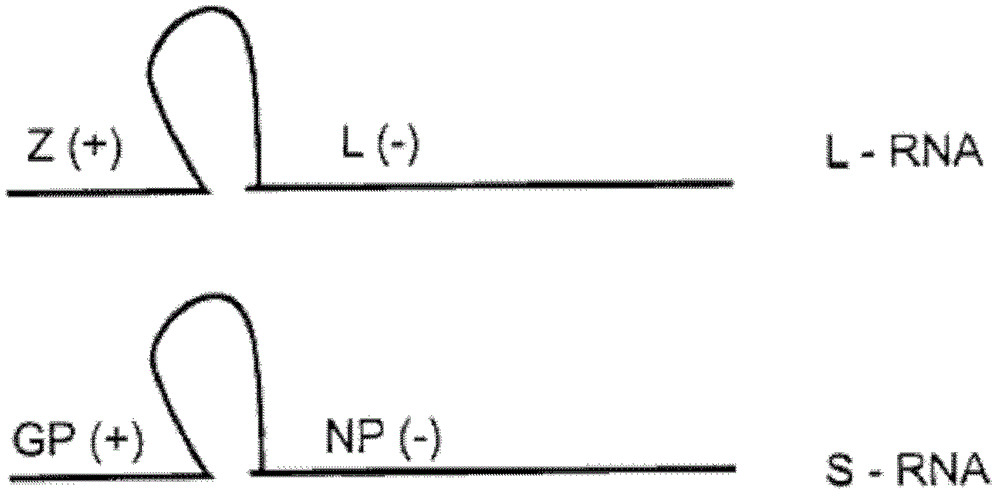
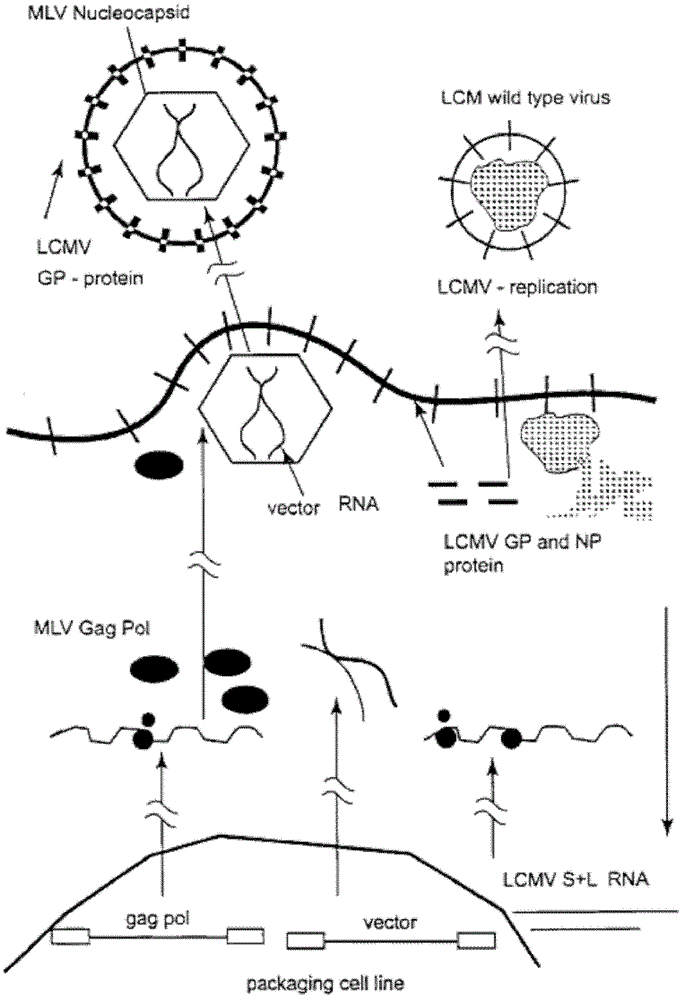
Lentiviral vector of pseudotyped lymphocytic choriomeningitis virus glycoprotein
A lymphocyte and choroid plexus technology, applied in the field of lentiviral vectors, can solve the problems of inability to achieve, loss of invasiveness of virus particles, difficulty in gene transfection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Infection of TeLCeb containing LCMV-LacZ gene transfers to target cells, neutralization of vector by concentration gradient of Anti-GP Mab and vector Use of LCMV to rescue envelope protein-negative murine leukemia virus vector: to prove whether LCMV can rescue envelope protein Negative murine leukemia virus vector, env-negative packaging cell line TELCeB was infected with LCMV WE line with an infectious multiplicity (m.o.i.: indicating the number of virions after cells were infected) of 0.01. TELCeB is derived from the human fibroblast cell line Te671 and contains gag, pol and the retroviral vector MFGnlsLacZ (G.M.Crooks et al., Blood 82 (1993) 3290-3297). After LCMV infection, the titer of LTU and LCMV multiplicity Type viruses were stained with X-gal of murine fibroblast target cells and plated (in plate-shaped units, PFU). In addition, the expression of LCMV glycoproteins in infected TELCeB can be measured by flow cytometry. The result is in. The LTU is 5x10 on the...
Embodiment 2
[0088] The products of the Gag and pol genes are required for packaging retroviral RNA import into the LCMV glycoprotein pseudotype model
[0089] Experiments were performed to confirm whether retroviral RNA alone can be packaged into LCMV or whether the products of the gag and pol genes are required to assist in the import. 293 cells and MLV293gp2 cells containing gag and pol were transfected with an MLV retroviral vector containing the neo gene (MP1N), and cell lines containing stable vector fragments were prepared by G418 selection (293MP1N and 293gp2MP1N; 293gp2MP1N cell line expressing SF23 Clones were deposited at DMSC - German Center for Microorganisms and Cells, Budapest protocol number DSM ACC2374). These cells were infected with replication-competent transfection cofactors or wild-type LCMV. The results are shown in Table 2. After infection with transfection cofactors, both cells can produce infectious vectors that transfer neomycin resistance. After infection wit...
Embodiment 3
[0094] LCMV Infection with 293gpMP1N and Stable Gene Transfer to L929 Cells by Southern Blotting MLV (LCMV) Pseudotype-Mediated Retroviral Vector Gene Transfer and Stable Integration: Resistance to G418 by a Retroviral LCMV Pseudotype Transfer, indicating that the target gene has been stably integrated into the host genome. To demonstrate that the MLV (LCMV) pseudonymous model mediates stable transduction by integrating genes into the host genome, a retroviral vector containing the neomycin repressor gene (neo) was infected by LCMV into the neo-negative enveloped cell line 293gp2MP1N to confirm. Titers were measured by shifting the inhibitory effect of G418 on Sc-1 cells in the range of 1x10 3 -1x10 4 between GTU / ml. Inhibitory cell clones emerged after 8 days and were cultured for an additional 3 weeks. Southern blot analysis of DNA from 12G418-resistant clones was performed with a neo probe after inhibition with monocutase HindIII. One gene copy of the integrated retrov...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com