Method for producing ferric chloride by using pickle liquor
A technology of pickling waste liquid and ferric chloride, which is applied in the direction of ferric halide, can solve the problems of high cost, large equipment investment, secondary pollution, etc., and achieve the effect of low production cost, safe operation and low investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
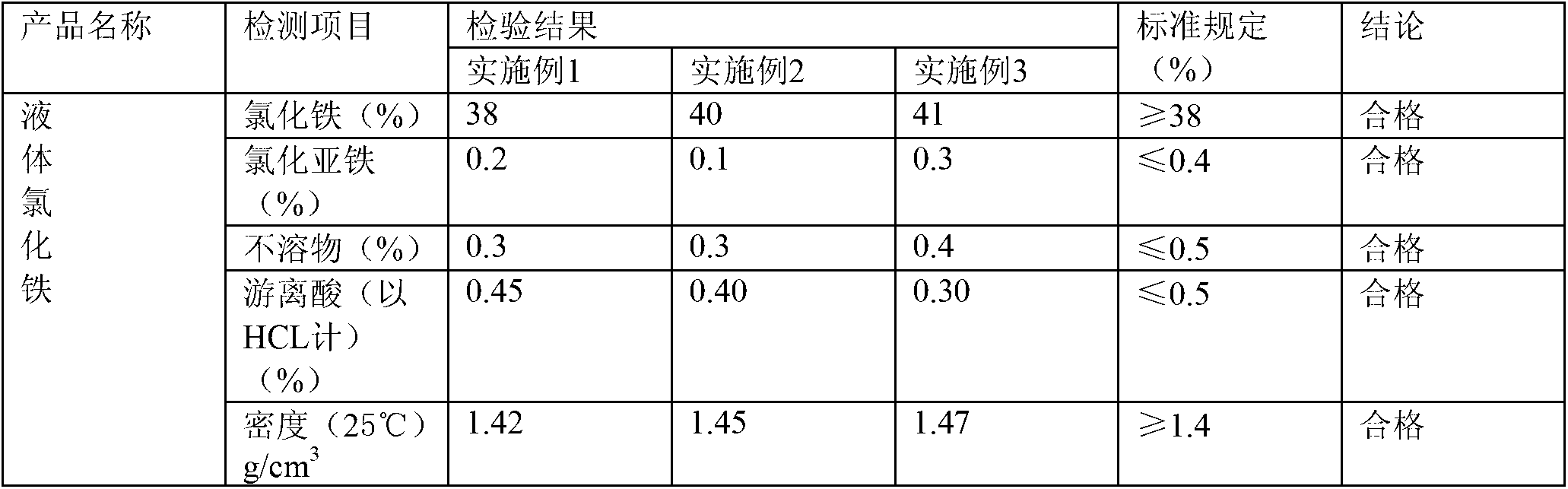
Examples
Embodiment 1
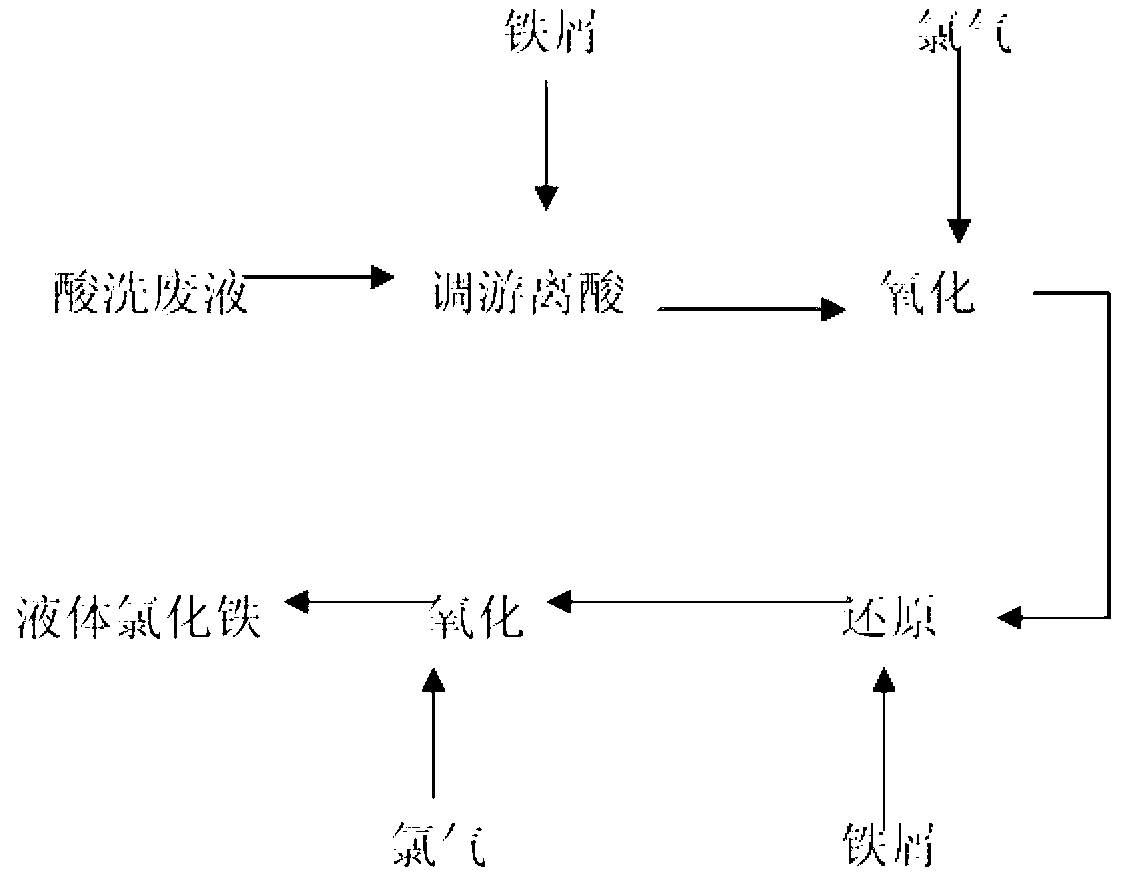
[0047] A method for producing ferric chloride by utilizing pickling waste liquor, is characterized in that, comprises the following steps:
[0048] (1) According to the iron content and hydrochloric acid content of the pickling waste liquid, add iron filings and soak for 32 hours to fully react the hydrochloric acid in the waste liquid;
[0049] (2) After soaking, pass chlorine gas to oxidize. Oxidation is carried out in a closed reaction kettle, each kettle is 5 tons, the temperature is controlled at 50~60℃, the flow rate of chlorine gas: 60~80kg / hour, the pressure control: 0.05~0.1MPa, and the time is 1h, oxidize the ferrous ions in the solution to ferric ions;
[0050] (3) After the oxidation reaction is finished, add iron filings so that the total iron content per ton of hot washing waste liquid is 130kg, control the temperature at 30-40°C, and soak for 24 hours;
[0051] (4) After soaking, pass chlorine gas to oxidize. Oxidation is carried out in a closed reaction kettle...
Embodiment 2
[0054] A method for producing ferric chloride by utilizing pickling waste liquor, is characterized in that, comprises the following steps:
[0055] (1) According to the iron content and hydrochloric acid content of the pickling waste liquid, add iron filings and soak for 48 hours to fully react the hydrochloric acid in the waste liquid;
[0056] (2) Pass chlorine gas to oxidize after immersion. Oxidation is carried out in a closed reaction kettle with 5 tons per kettle. 3h, the divalent iron ions in the solution are oxidized to ferric ions;
[0057] (3) After the oxidation reaction is finished, add iron filings so that the total iron content per ton of pickling waste liquid is 135kg, control the temperature at 30-40°C, and soak for 24 hours;
[0058] (4) After soaking, pass chlorine gas to oxidize. Oxidation is carried out in a closed reaction kettle, each kettle is 5 tons, the temperature is controlled at 50~60℃, the flow rate of chlorine gas: 60~70kg / hour, the pressure cont...
Embodiment 3
[0061] A method for producing ferric chloride by utilizing pickling waste liquor, is characterized in that, comprises the following steps:
[0062] (1) According to the iron content and hydrochloric acid content of the pickling waste liquid, add iron filings and soak for 60 hours to fully react the hydrochloric acid in the waste liquid;
[0063] (2) Pass chlorine gas to oxidize after immersion. Oxidation is carried out in a closed reaction kettle with 5 tons per kettle. 3h, the divalent iron ions in the solution are oxidized to ferric ions;
[0064] (3) After the oxidation reaction is finished, add iron filings so that the total iron content per ton of pickling waste liquid is 140kg, control the temperature at 30-40°C, and soak for 24 hours;
[0065] (4) After soaking, pass chlorine gas to oxidize. Oxidation is carried out in a closed reaction kettle, each kettle is 5 tons, the temperature is controlled at 50~60℃, the flow rate of chlorine gas: 60~70kg / hour, the pressure cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com