Preparation method of 2-arylbenzimidazole
A technology of benzimidazole and aryl group, applied in the field of preparing 2-arylbenzimidazole, can solve the problems of complicated post-processing process, high catalyst cost, long reaction time and the like, and achieves short reaction time, good catalytic effect and low cost low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
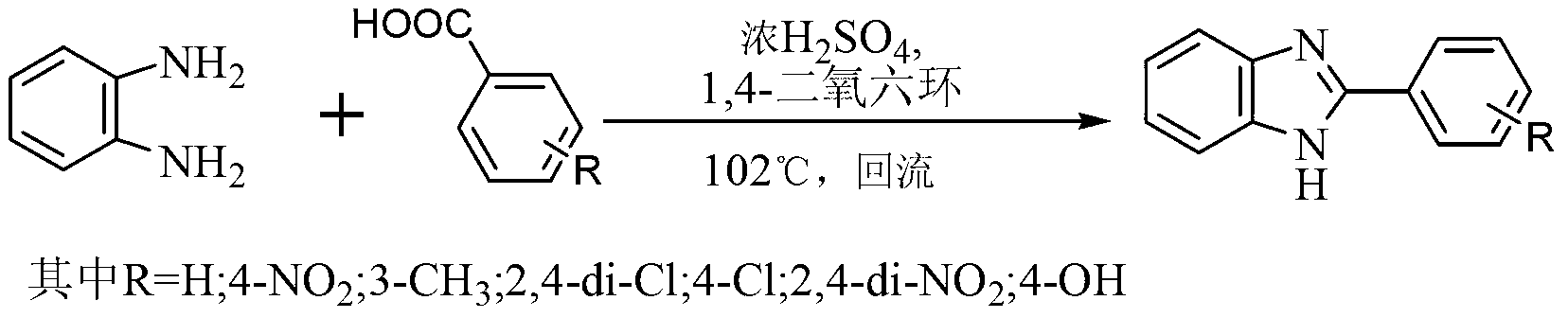
[0022] 1) Add 0.01mol of o-phenylenediamine and 0.01mol of benzoic acid to a dry three-necked flask, then add 5mL of 1,4-dioxane, and then add 0.001mol of concentrated sulfuric acid with a mass concentration of 98% dropwise under stirring , kept at 102°C and refluxed for 5 hours under sufficient stirring to obtain a mixed solution;
[0023] 2) Cool the mixed solution to room temperature, then add NaOH aqueous solution with a mass concentration of 10% to the mixed solution to make the mixed solution alkaline and precipitate solids, then filter the mixed solution under reduced pressure, and air-dry the obtained solid naturally, that is 2-Phenylbenzimidazole was obtained in a yield of 76%.
[0024] The present invention further provides the comparative example of embodiment 1, promptly does not add the preparation method of concentrated sulfuric acid catalyst and prepares 2-phenylbenzimidazole, and its preparation method is as follows:
[0025] 1) Add 0.01mol of o-phenylenediami...
Embodiment 2
[0029] 1) Add 0.01mol of o-phenylenediamine and 0.011mol of benzoic acid to a dry three-necked flask, then add 5mL of 1,4-dioxane, and then add 0.001mol of concentrated sulfuric acid with a mass concentration of 98% dropwise under stirring , keep reflux at 102°C for 4.5h under sufficient stirring to obtain a mixed solution;
[0030] 2) Cool the mixed solution to room temperature, then add NaOH aqueous solution with a mass concentration of 10% to the mixed solution to make the mixed solution alkaline and precipitate solids, then filter the mixed solution under reduced pressure, and air-dry the obtained solid naturally, that is 2-Phenylbenzimidazole was obtained with a yield of 85%.
Embodiment 3
[0032] 1) Add 0.01mol of o-phenylenediamine and 0.012mol of benzoic acid to a dry three-necked flask, then add 5mL of 1,4-dioxane, and then add 0.001mol of concentrated sulfuric acid with a mass concentration of 98% dropwise under stirring , keep reflux at 102°C for 4h under sufficient stirring to obtain a mixed solution;
[0033] 2) Cool the mixed solution to room temperature, then add NaOH aqueous solution with a mass concentration of 10% to the mixed solution to make the mixed solution alkaline and precipitate solids, then filter the mixed solution under reduced pressure, and air-dry the obtained solid naturally, that is 2-Phenylbenzimidazole was obtained in 90% yield, IR(υ max , KBr, cm -1 ): 1625(-C=N), 3190(-NH); 1 HNMR (δppm, CDCl 3 ):12.96(s,1H,-NH),7.94(d,2H,Ar-H),7.25-7.27(m,4H,Ar-H),7.41-7.44(m,1H,Ar-H),6.90 -6.92 (m, 2H, Ar-H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com