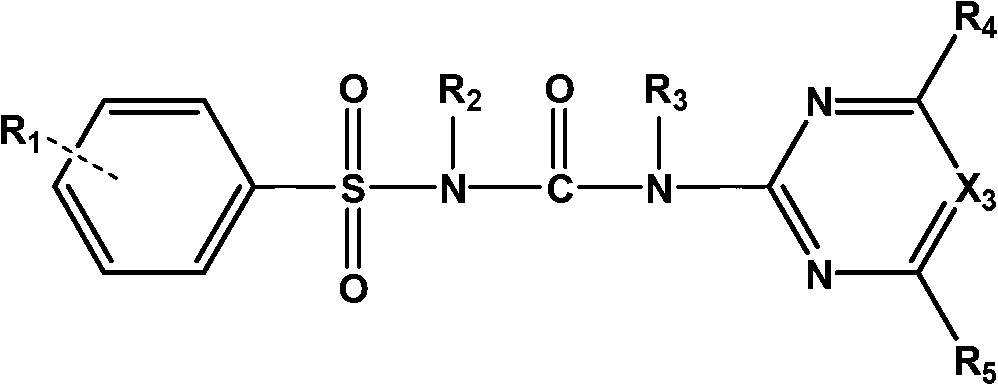
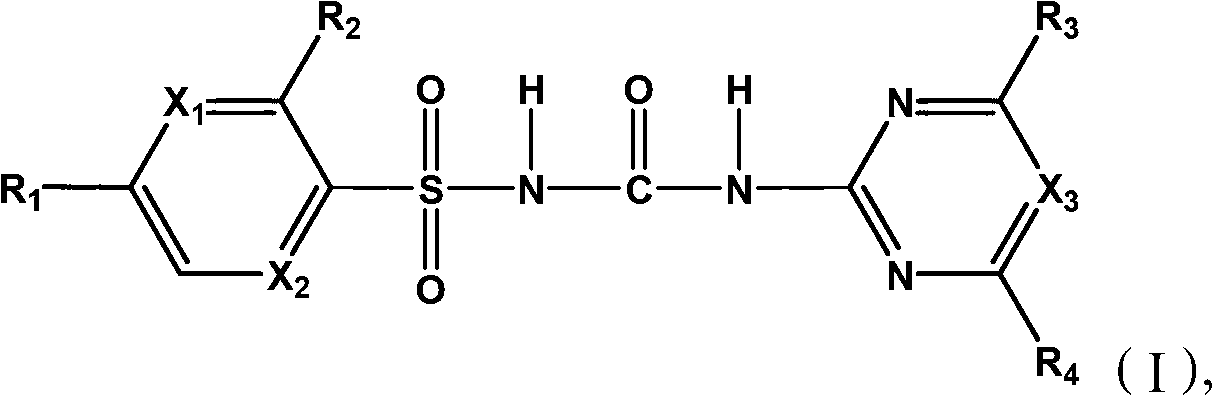
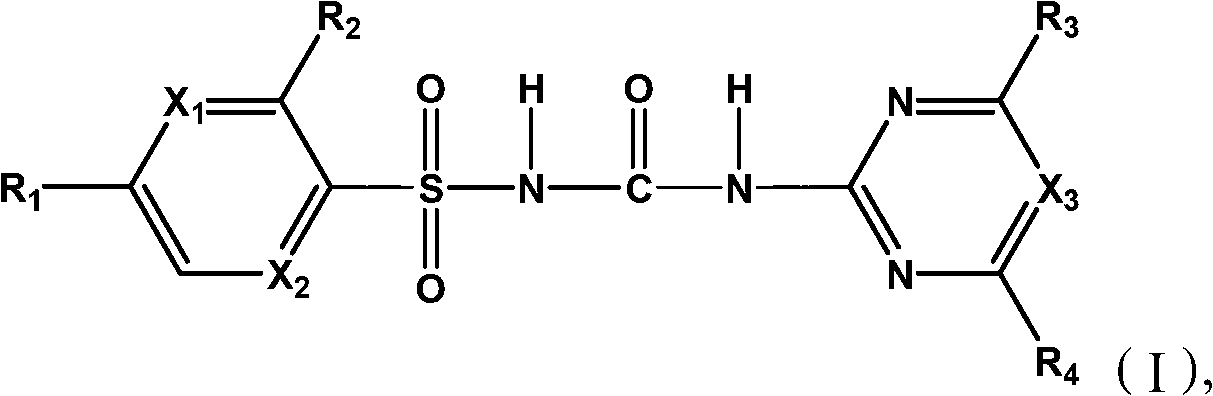
Sulfonylurea compound
A technology for sulfonylureas and compounds, applied in the field of organic compounds, can solve the problems of reduced herbicidal activity and selectivity, and achieve the effects of low residue, low residue and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of (2-methoxycarbonyl-4-nitro)phenyl tert-butyl sulfide
[0034] Take a 250mL round bottom flask, put it into an oil bath, set the temperature of the oil bath to 25°C, and 2 Under atmosphere, add 10mL DMSO, 2.4g KOH, stir, after 20min, add 6mL tert-butylmercaptan to the round bottom flask, and continue stirring for 30min. Add 7.84g of methyl 2-chloro-5-nitrobenzoate to the round bottom flask, set the temperature of the oil bath to 110°C after the addition is complete, a yellow solid is formed during the heating process, and react for 90 minutes after the temperature reaches 110°C . The heating was stopped, and the flask was cooled to room temperature, and there was a yellow-black solid at the bottom of the bottle. Add 150mL of water to the round bottom flask, a yellow solid precipitates, stir, filter with suction, wash with water to obtain a yellow solid, extract the filtrate with 2×200mL ethyl acetate, combine the ethyl acetate filtrate, and wash with 2×100...
Embodiment 2
[0043] Adopt existing method to prepare following sulfonylurea compounds:
[0044]
[0045] NMR hydrogen spectrum (TMS is internal standard, CDCl 3 is the solvent) shows that the compound contains 10 hydrogens, and the peak is assigned to 12.225 (s 1H SO 2 NH)8.330~8.353(m2HArH)8.384~8.407(m2HArH)7.310(s1HCONH)6.513(s1HArH)4.012(s3HOCH 3 ).
Embodiment 1 and 2
[0047] The herbicidal activity experiment of sulfonylurea compound A and B in embodiment 1 and 2:
[0048] Plant barnyardgrass seeds in the seed germination box, spray with 1ppm of A and B herbicides after 5 days of germination, the results show that the sulfonylurea compound with general formula (I) has high herbicidal activity, and the product meets the requirements of environmental friendliness .
[0049] Table 2 Herbicidal activity of target compounds 1 and 3
[0050]
[0051]
[0052] ++++ means that all the branches and leaves are scorched, and there are no new sprouts;
[0053] +++ indicates that the branches and leaves are withered and yellow, and there are no new sprouts;
[0054] ++ indicates that the branches and leaves are withered and yellow, and there are a small amount of newly sprouted leaves;
[0055] + means only a few branches and leaves are withered and yellow, and there are more new green branches and leaves
[0056] 0 There are more new green br...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com