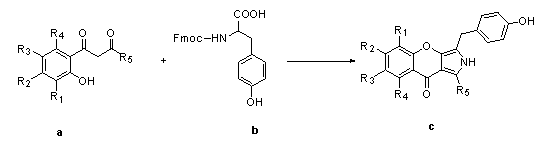
Substituted pyrrole chromone compound
A pyrrole chromone and compound technology, applied in organic chemistry, drug combination, cardiovascular system diseases, etc., can solve problems such as adverse reactions, digestive disorders, and congestion in patients with liver and kidney insufficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
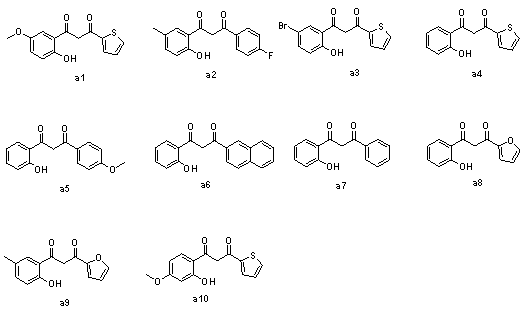
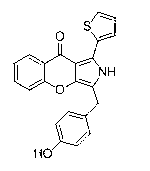
[0050] 1mmol of 1-(thienyl-2-)-3-(2-hydroxy-5-methoxyphenyl)propane-1,3-dione (a1) was dissolved in 1.8mmol Fmoc-Tyr(tBu)-OH In 10ml of pyridine, add 2mmol N,N-dicyclohexyldiimine, add 0.6mmol N,N-lutidine, stir at room temperature for about 3h, until TLC detects that raw material a1 disappears, heat up to 50°C to react 4- 6h to produce a predominantly yellow dot. After the reaction was completely evaporated to dry pyridine, ethyl acetate was added, N,N-dicyclohexyl urea would precipitate out, suction filtered, and the filtrate was obtained by column chromatography P0-1 , yellow solid, yield is 77%, its structure and NMR data are as follows:
[0051]
[0052] 1 H NMR (400 MHz, DMSO) δ 12.35 (1H), 8.02 (d, J = 3.5 Hz, 1H), 7.57 (dd, J = 13.4, 3.9 Hz, 2H), 7.43 (d, J = 9.1 Hz, 1H), 7.31 (dd, J = 9.0, 3.0 Hz, 1H), 7.20 – 7.13 (m, 3H), 6.91 (d, J = 8.4 Hz, 2H), 4.11 (1H), 3.85 (3H), 1.25 (9H); 13 C NMR (101 MHz, CDCl 3 ) δ 175.34, 155.11, 154.23, 151.58, 142.37, 1...
Embodiment 2
[0057] 1 mmol 1-(4-fluorophenyl)-3-(2-hydroxy-5-methylphenyl)propane-1,3-dione (a2) with 1.8 mmol Fmoc-Tyr(tBu)-OH, 2 mmol N , N-dicyclohexyldiimine, 0.6mmol N, N-lutidine, according to the method of Example 1, to obtain P0-2 , a yellow solid, which was directly used in the next step of synthesis. The structure is as follows:
[0058]
[0059] compound P0-2 Process according to the method of embodiment 1, obtain P2 , yellow solid, yield 56%, its structure and NMR data are as follows:
[0060]
[0061] 1 H NMR (400 MHz, DMSO) δ 12.30 (1H), 9.20(1H), 8.13 (s, 2H), 7.94 (s, 1H), 7.51 (d, J = 8.2 Hz, 1H), 7.37 (d, J = 8.3 Hz, 1H), 7.30 (t, J = 8.3 Hz, 2H), 7.10 (d, J = 7.6 Hz, 2H), 6.70 (d, J = 7.7 Hz, 2H), 4.04(2H), 2.40(3H); 13 C NMR (101 MHz, DMSO) δ 174.17, 162.79, 160.34, 155.69, 154.21, 141.53, 134.87, 131.74, 129.86, 129.78, 129.58, 128.96×2, 127.56, 127.54, 126.09, 125.80, 121.54, 117.22, 115.18×2 , 115.07, 114.86, 113.50, 107.28, 28.43, 20.29.
Embodiment 3
[0063] 1 mmol of 1-(thienyl-2-)-3-(2-hydroxy-5-bromophenyl)propane-1,3-dione (a3) with 1.8 mmol of Fmoc-Tyr(tBu)-OH, 2 mmol of N , N-dicyclohexyldiimine, 0.6mmol N, N-lutidine, according to the method of Example 1, obtained P0-3 , a yellow solid, which was directly used in the next step of synthesis. The structure is as follows:
[0064]
[0065] compound P0-3 Obtain according to the method of embodiment 1 P3 , yellow solid, yield 32%, its structure and NMR data are as follows:
[0066]
[0067] 1 H NMR (400 MHz, DMSO) δ 12.41 (1H), 9.21(1H), 8.20 (d, J = 2.4 Hz, 1H), 8.02 (d, J = 3.2 Hz, 1H), 7.84 (dd, J = 8.8, 2.4 Hz, 1H), 7.58 (d, J = 4.9 Hz, 1H), 7.45 (d, J = 8.9 Hz, 1H), 7.19 – 7.14 (m, 1H), 7.08 (d, J = 8.3 Hz, 2H), 6.70 (d, J = 8.3 Hz, 2H), 4.04 (2H); 13 C NMR (101 MHz, DMSO) δ 172.39, 155.73, 155.06, 140.87, 136.41, 132.88, 129.29, 128.94×2, 128.35, 127.37, 126.43, 123.59, 122.08, 120.11, 115.20×2, 114.71, 113.69, 106.51, 28.28 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com