Fulvestrant containing oil needle preparation
A technology of fulvestrant and oil injection, applied in the field of non-aqueous ester solvent, fulvestrant injectable oil injection preparation, intramuscular injection oil injection preparation, excipient soybean oil, which can solve irritation, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
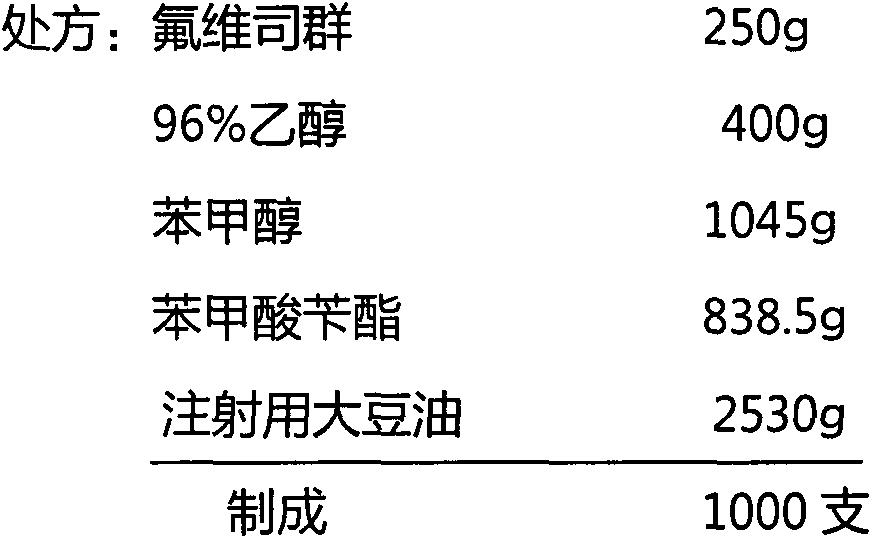
[0016] Preparation of oil injection preparation of fulvestrant (50mg / ml)
[0017]
[0018] Weigh the 96% ethanol and benzyl alcohol of the prescribed amount to mix, add the fulvestrant of the prescribed amount, stir and dissolve.
[0019] Add the prescribed amount of benzyl benzoate, stir, and mix well.
[0020] Add the soybean oil for injection of prescription quantity to make preparation solution into final weight, stir, mix homogeneously.
[0021] Add 5 g of activated carbon, stir at 25°C for 30 minutes, filter through a 0.45μm filter membrane to remove carbon, and then filter through a 0.22μm filter membrane to sterilize.
[0022] Determination of intermediate content.
[0023] Under aseptic conditions, the filtrate was filled in 5ml ampoule bottles with a filling volume of 5.25ml, covered with sterile nitrogen gas, and sealed by fusing.
Embodiment 2
[0025] Preparation of oil injection preparation of fulvestrant (50mg / ml)
[0026]
[0027] Weigh the 96% ethanol and benzyl alcohol of the prescribed amount to mix, add the fulvestrant of the prescribed amount, stir and dissolve.
[0028] Add the prescribed amount of benzyl benzoate, stir, and mix well.
[0029] Add the soybean oil for injection of prescription quantity to make preparation solution into final weight, stir, mix homogeneously.
[0030] Add 5 g of activated carbon, stir at 25°C for 30 minutes, filter through a 0.45μm filter membrane to remove carbon, and then filter through a 0.22μm filter membrane to sterilize.
[0031] Determination of intermediate content.
[0032] Under aseptic conditions, the filtrate was filled in 5ml ampoule bottles with a filling volume of 5.25ml, covered with sterile nitrogen gas, and sealed by fusing.
Embodiment 3
[0033] Example 3: Study on the Stability of Oil Injection Preparation of Fulvestrant
[0034] In accordance with the quality standards for the application of fulvestrant oil injection preparations for clinical research and the investigation items listed in the stability test of injections in the second appendix of the Chinese Pharmacopoeia 2010 edition "Guiding Principles for Drug Stability Tests", we stabilized this product. sex inspection.
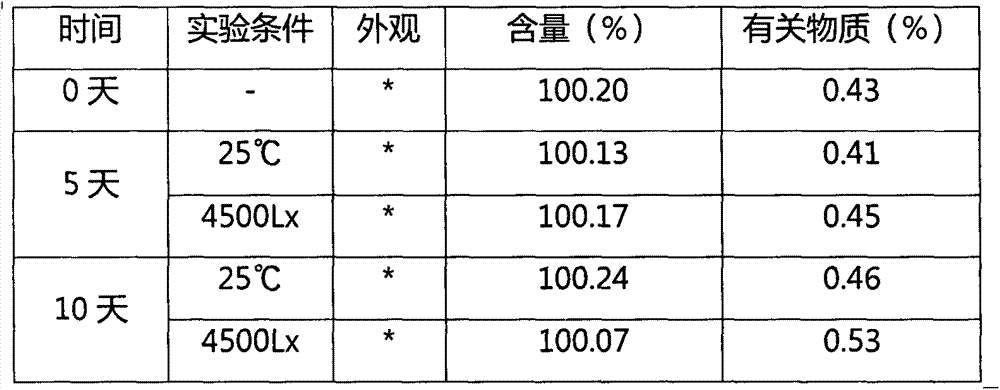
[0035] Influencing factor test
[0036] Influencing factor test method: Take this product (batch number: 20120521), remove the outer packaging, and place it at 25°C and light intensity of 4500Lx for 10 days, and take samples for inspection on the 5th and 10th days respectively. The content of vestrant and related substances are the main indicators of investigation, and the stability of the preparation to temperature and light is compared with the data of 0 days. The results are shown in Table 1.
[0037] Table 1 Influencing factor tes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com