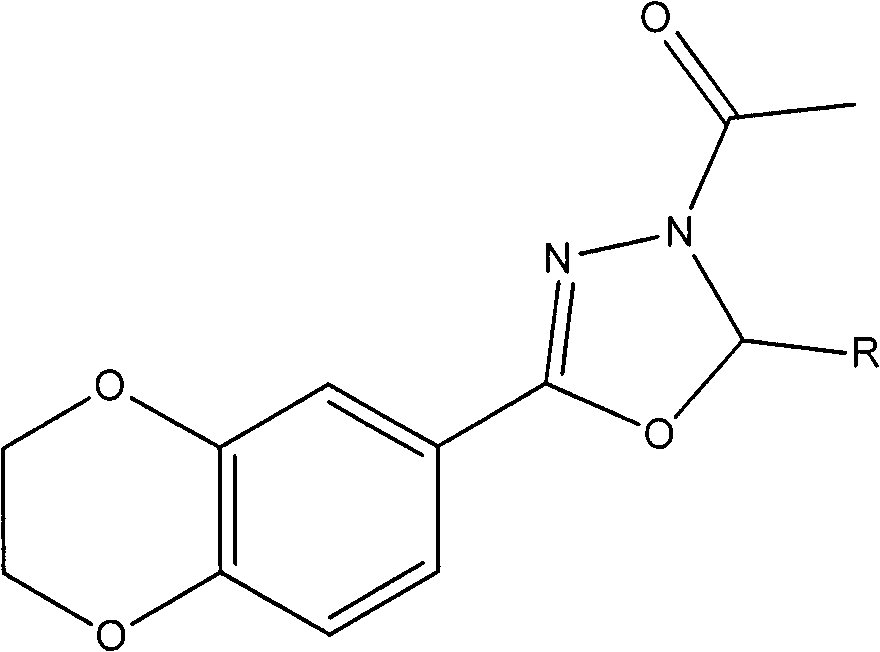
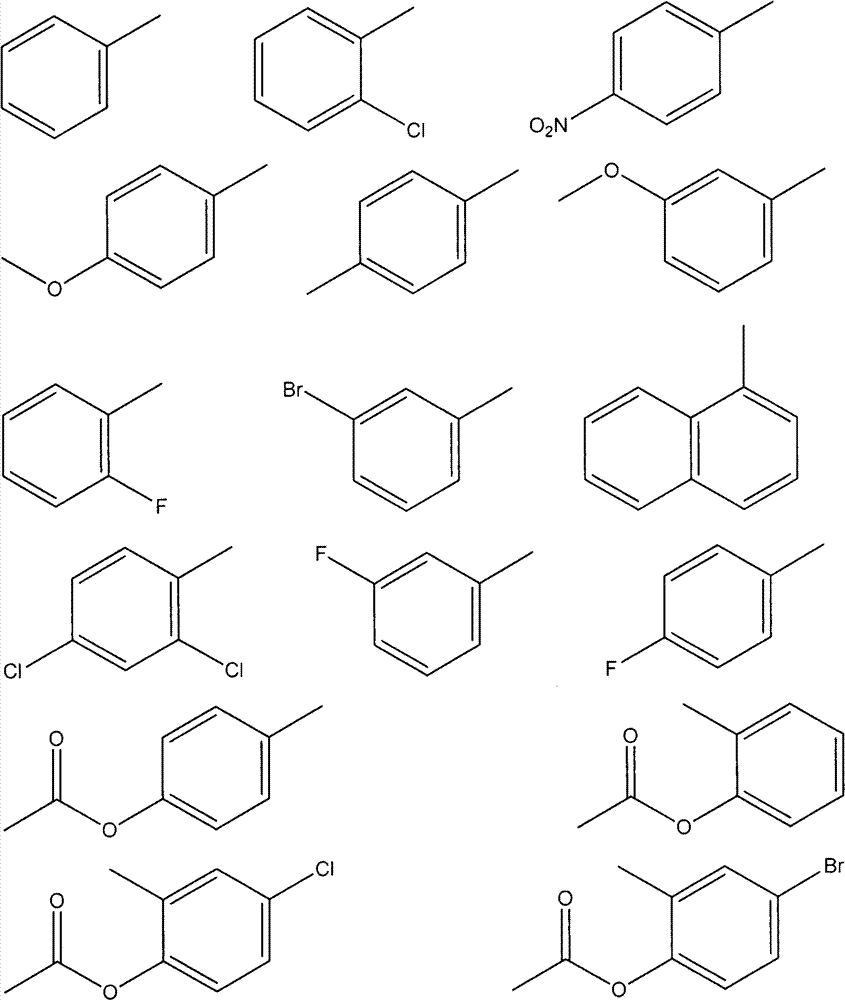
Preparation of oxadiazole compound and application thereof to anti-inflammatory treatment
A technology of oxadiazoles and target compounds, applied in anti-inflammatory agents, organic chemistry, drug combination, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: 2-phenyl-3-acetyl-5-(2,3-dihydrobenzo[b][1,4]dioxane-6-yl)-1,3,4-oxa Preparation of Oxadiazole (Compound 5a)
[0024]
[0025] Take 0.0015moL of hydrazide and equimolar carbonyl compounds and add them to 50mL of round bottom burner, add 30mL of absolute ethanol and 7.5mL of water, heat to dissolve, add 0.3mL of glacial acetic acid, and reflux for 5h. After the reaction, it was poured into water, and the precipitated solid was filtered, washed with water, dried and then recrystallized by absolute ethanol to obtain Schiff's base. Take 0.001 mol of Schiff's base and equimolar benzaldehyde after drying and put them into a 25 mL round bottom flask, add 5 mL of acetic anhydride and 2 mL of glacial acetic acid, heat to reflux for 1 hour, pour into ice water after cooling, and stir until solids precipitate out , the precipitated solid was filtered, washed with water, dried and recrystallized with absolute ethanol to obtain pure compound 4a (white crystal). Yield...
Embodiment 2
[0026] Example 2: 2-(2-chlorophenyl) 3-acetyl 5-(2,3-dihydrobenzo[b][1,4]dioxane-6-yl)-1,3, Preparation of 4-oxadiazole (compound 5b)
[0027]
[0028] The preparation method is the same as in Example 1. The benzaldehyde in Example 1 was replaced with LinCl benzaldehyde to obtain the target compound in the form of white crystals. White crystal, Yield 69%, mp: 171-172°C, 1H NMR (300MHz, DMSO-d6) δ: 2.26(s, 3H), 4.29-4.30(m, 4H), 6.98(d, J=8.4Hz, 1H), 7.22(d, J=2.0Hz, 1H), 7.28-7.31(m, 2H), 7.41-7.51(m, 3H), 7.56(d, J=7.9Hz, 1H).MS(ESI): 359.8 (C 18 h 16 ClN 2 o 4 ,[M+H]+).Anal.Calcd for C 18 h 15 ClN 2 o 4 : C, 60.26; H, 4.21; N, 7.81. Found: C, 60.24; H, 4.21; N, 7.83.
Embodiment 3
[0029] Example 3: 2-(4-nitrophenyl)-3-acetyl-5-(2,3-dihydrobenzo[b][1,4]dioxane-6-yl)-1 , Preparation of 3,4-oxadiazole (compound 5c)
[0030]
[0031] The preparation method is the same as in Example 1. The benzaldehyde in Example 1 was replaced by p-nitrobenzaldehyde to obtain the target compound in the form of yellow crystals. Yield 60%; mp: 151-152°C 1H NMR (DMSO-d 6 , 300MHz): 2.25(s, 3H), 4.28-4.31(m, 4H), 7.01(d, J=8.40Hz, 1H), 7.27-7.31(m, 2H), 7.32-7.36(m, 1H), 7.76 (d, J=8.79Hz, 2H), 8.29 (d, J=8.61Hz, 2H). MS (ESI): 370.10 (C18H16N3O6, [M+H]+). Anal. Calcd for C18H15N3O6: C, 58.54 ; H, 4.09; N, 11.38; O, 25.99. Found: C, 58.53; H, 4.09; N, 11.40.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com