Solid pharmaceutical composition containing benzimidazole derivative
A composition and drug technology, applied in the field of preparation of antihypertensive drugs, can solve the problems of failure to prove the dissolution advantage and limited improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
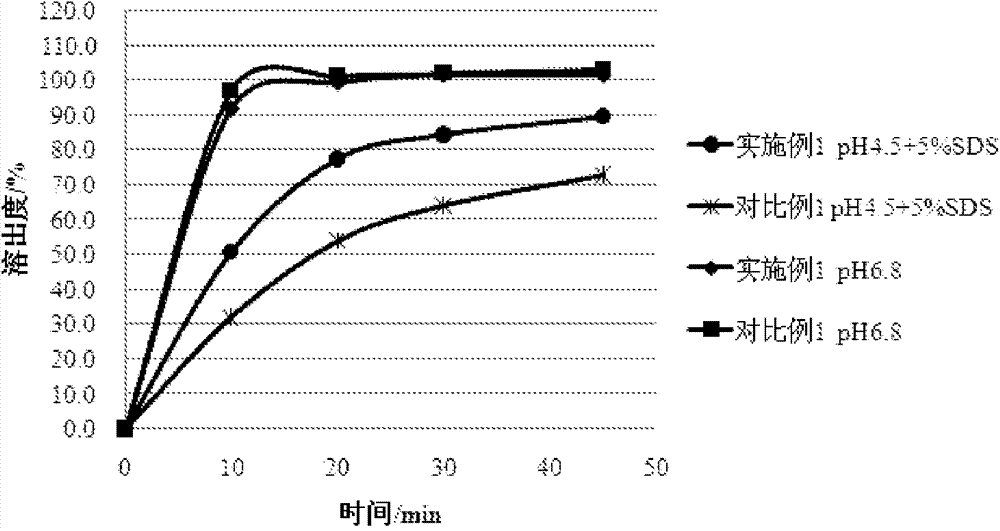
Embodiment 1
[0032] Azilsartan (64g) treated by jet milling (d(0.5)=2.61μm, d(0.9)=5.24μm), mixed with mannitol (200g), microcrystalline cellulose (30g), croscarmellose Sodium cellulose cellulose (16g) was mixed evenly, and 5% hydroxypropyl cellulose aqueous solution was used as a binding agent, granulated, dried in a fluidized bed, and granulated with a 1.0mm sieve. Add 3.3 g of magnesium stearate to the sized granules, and mix well. The resulting mixture was tabletted through a 7.0 mm punch to obtain a plain tablet having the following composition.
[0033] Composition of preparation (per 159.6mg)
[0034]
[0035]
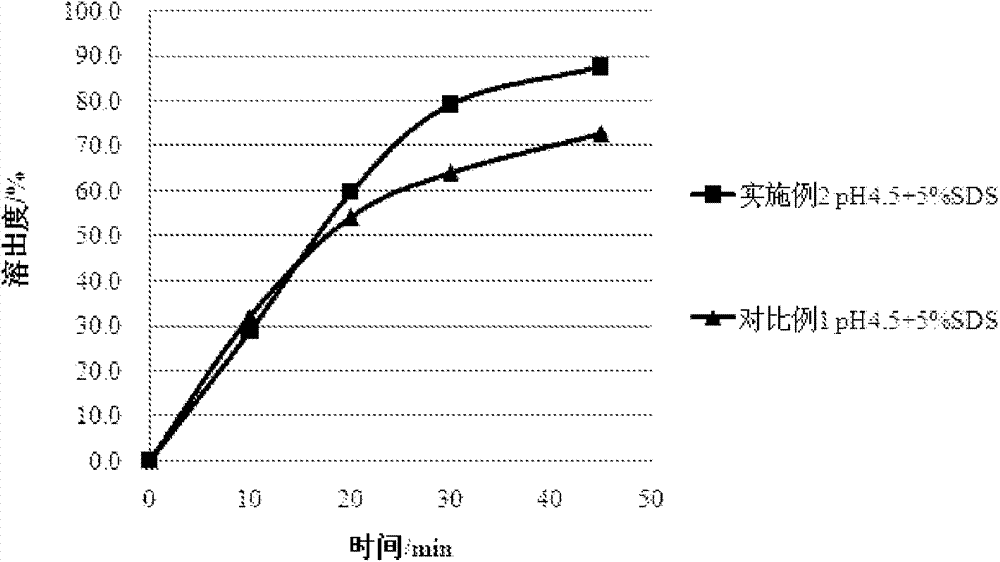
Embodiment 2
[0037] Mix azilsartan (64g) and β-cyclodextrin (384g) uniformly, add 768g of water, grind for 6 hours until it becomes semi-solid, and dry under reduced pressure at 40°C to obtain a solid. The obtained solid was washed with appropriate amount of water and methanol, and dried under reduced pressure to obtain clathrate. Take appropriate amount of clathrate (containing 32g of azilsartan), mix well with mannitol (100g), microcrystalline cellulose (15g), croscarmellose sodium (8g), and use 5% hydroxypropyl cellulose The plain aqueous solution is used as a binder, granulated, fluidized bed dried, and granulated with a 1.0mm sieve. Add 3.3 g of magnesium stearate to the sized granules, and mix well. The resulting mixture was tabletted through a 10.0 mm punch to obtain a plain tablet having the following composition.
[0038] Composition of preparation (per 345.5mg)
[0039]
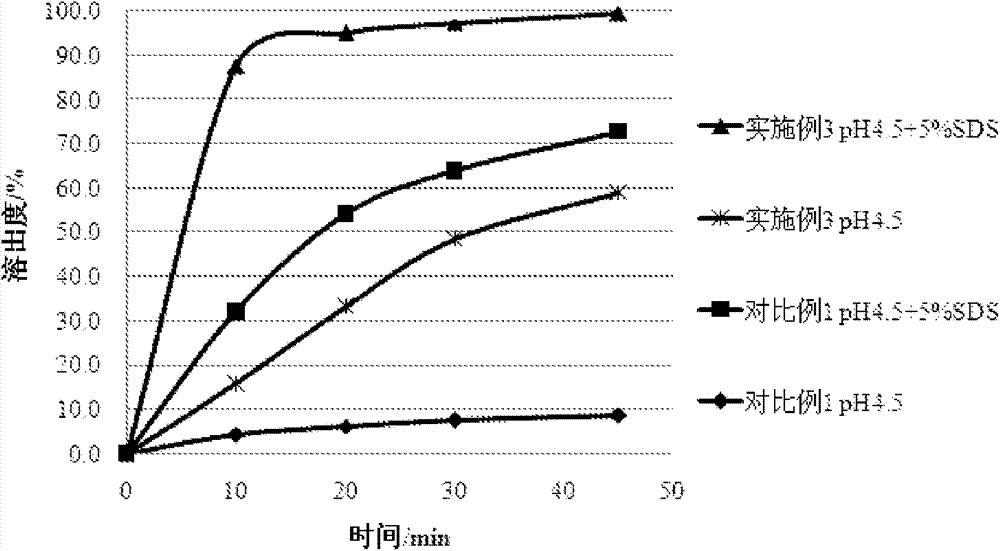
Embodiment 3
[0041] Azilsartan (64g), mixed with mannitol (90g), microcrystalline cellulose (90g), croscarmellose sodium (15g), sodium carbonate (40g), was mixed with 5% hydroxypropyl The cellulose aqueous solution is used as a binder, granulated, fluidized bed dried, and granulated with a 1.0mm sieve. Add 3 g of magnesium stearate to the sized granules, and mix well. The resulting mixture was tabletted through a 7.0 mm punch to obtain a plain tablet having the following composition.
[0042] Composition of preparation (per 156mg)
[0043]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com