Methods of generating natural killer cells
A natural killing, cell technology, applied in biochemical equipment and methods, animal cells, vertebrate cells, etc., can solve the problems of difficult to maintain tumor targeting and killing ability, difficult to apply immunotherapy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0268] 7.1. Example 1: Recovery of hematopoietic stem cells from human placental perfusate and umbilical cord blood
[0269] Human placental perfusate (HPP) and umbilical cord blood (UCB) cells are usually purified using Ficoll or ammonium chloride to obtain total nucleated cells (TNC). TNCs were then used in a positive selection procedure using anti-CD34 beads and RoboSep according to the manufacturer's (StemCell Technologies, Inc.) protocol to isolate CD34 + cell. In this experiment, CD34 was isolated with a purity greater than 90% + cell. As another option, the Human Progenitor Cell Enrichment Kit (StemCell Technologies, Inc.) was developed by using the Human Progenitor Cell Enrichment Mixture and the following human cell surface antigens: CD2, CD3, CD11b, CD11c, CD14, CD16, CD19, CD24, CD56, CD66b and glycophorin A monoclonal antibodies were used in a negative selection procedure to exclude lineage-committed cells. Using negative selection, 90% of CD34 was recovere...
Embodiment 2
[0272] 7.2. Example 2: Feeder-free Expansion and Differentiation of Hematopoietic Stem Cells to Natural Killer Cells
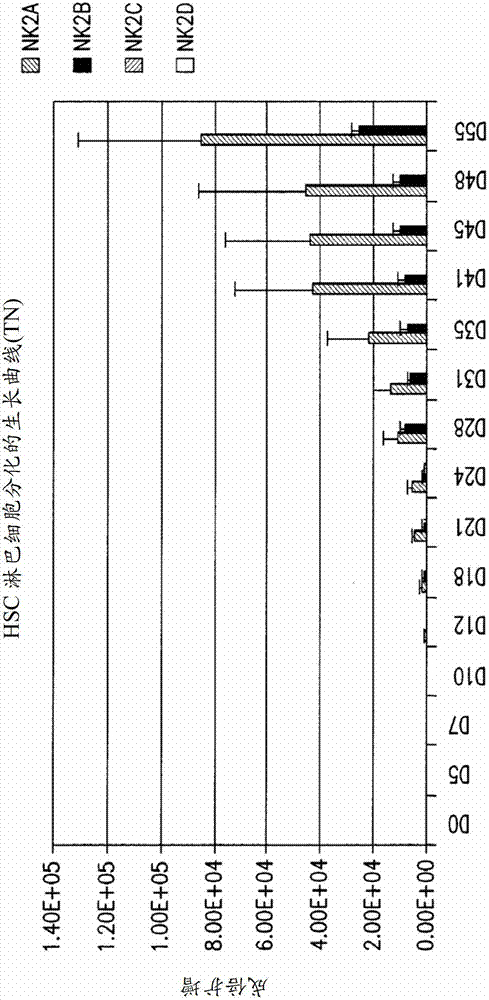
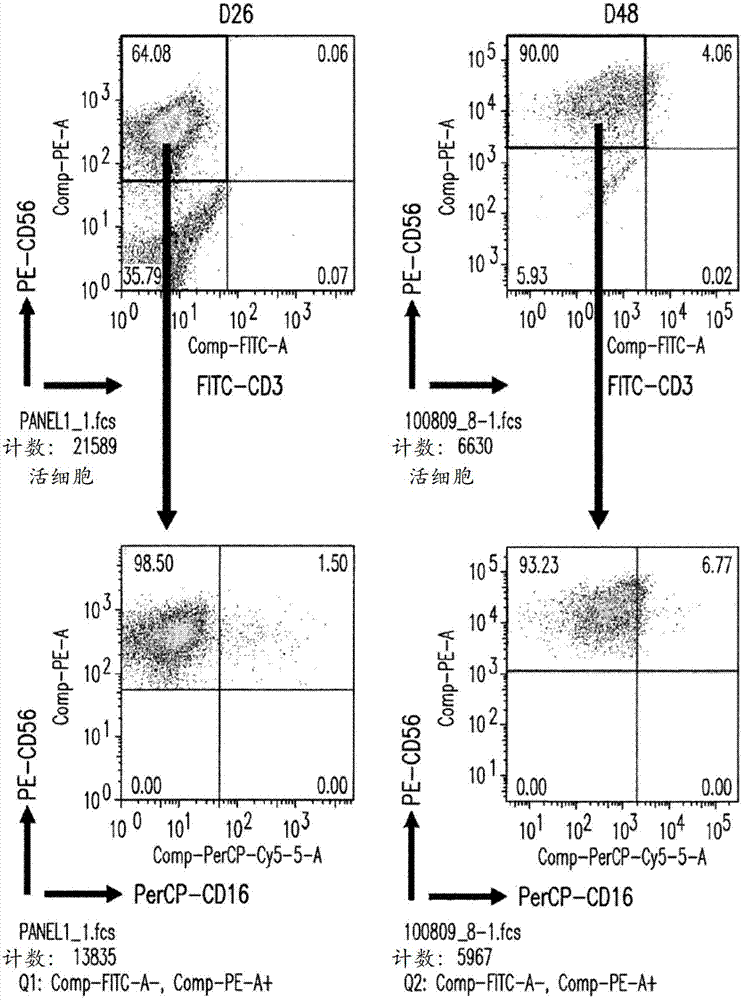
[0273] CD34 + Cells were cultured in the following media formulations for up to 48 days, and cell aliquots were taken for cell counts, assessment of cell viability, identification of natural killer cell differentiation, and functional assessment.
[0274] NK1 medium : GBGM (Glycostem Basal Growth Medium, Glycostem Cat. No.: CCT-SCB500, Clear cell technology) supplemented with pen / strep (Cat. No.: 15140, Gibco), 20 ng / niL SCF (Cat. No.: 255-SC, R&D Systems), 10ng / mL Flt-3 Ligand (Cat. No.: 308-FK, R&D system), 20ng / mL TPO (Cat. No.: 288-TP, R&D system), 20ng / mL IL-7 (Cat. No.: 207 -IL, R&D Systems), 200IU / mL IL-2 (catalogue number: 202-IL, R&D Systems), and 10ng / mL IL-15 (catalogue number: 247-IL, R&D Systems).
[0275] NK2 medium : DMEM (Cat. No.: MT-10-013-CV, Fisher): Ham's F12 medium (Cat. No.: BW12-615F, Fisher) (1:2) supplemented with 2 mM L-gluta...
Embodiment 3
[0295] 7.3. Embodiment 3: the culture of NK cell in CNK culture medium
[0296] Enhance NK cell expansion and cytotoxicity
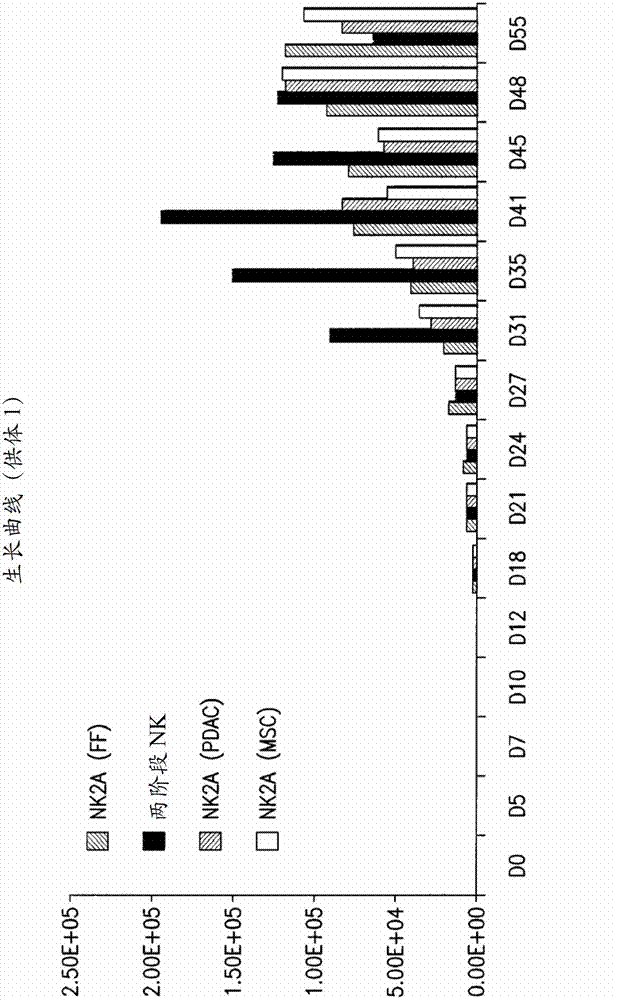
[0297] On day 27 (D27), CD34 cultured in NK2A medium + Cells were further cultured in one of the following media:
[0298] • A two-stage medium comprising CNK medium and maintenance medium. The CNK medium was IMDM (Invitrogen) supplemented with 10% FCS (Hyclone), 200 IU / mL IL-2 (R&D Systems), 35 μg / mL transferrin (Sigma-Aldrich), 5 μg / mL insulin (Sigma-Aldrich ), 2×10-5M ethanolamine (Sigma-Aldrich), 1 μg / mL oleic acid (Sigma-Aldrich), 1 μg / mL linoleic acid (Sigma-Aldrich), 0.2 μg / mL palmitic acid (Sigma-Aldrich), 2.5 μg / mL BSA (Sigma-Aldrich) and 0.1 μg / mL phytohemagglutinin (PHA-P, Sigma-Aldrich). CD56 cultured in NK2A medium + CD3 - NK cells were resuspended at 2.5×l05 viable cells / mL in CNK medium in cell culture-treated 24-well plates or square flasks. Allogeneic PBMC and K562 cells (chronic myelogenous leukemia cell line) treated with mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com