Liposome, a pharmaceutical composition comprising the liposome, and a method of delivering active agents to a target site using the liposome
A technology of liposomes and active agents, applied in drug combination, liposome delivery, drug delivery, etc., can solve problems such as limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1: Preparation of liposomes and measurement of thermal sensitivity
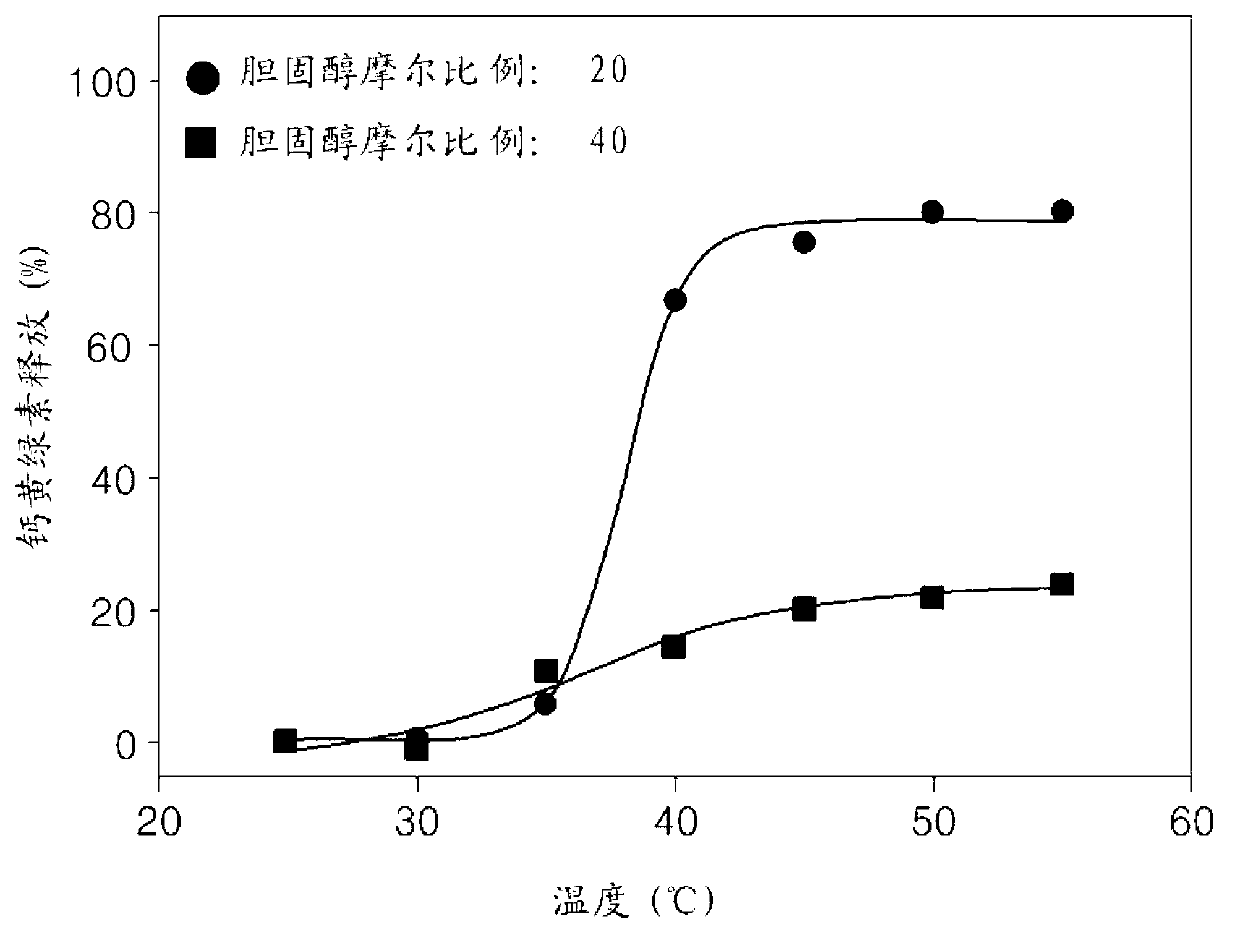
[0080] Use stearoyl-VPGVG VPGVG VPGVG VPGVG VPGVG VPGVG-NH at a molar ratio of 0.55:55:2:20 or 0.55:55:2:40 2 (SEQ NO:6, hereinafter referred to as "SA-V6-NH 2 ”), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), [1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy( Polyethylene glycol)-2000] (ammonium salt)] (DSPE-PEG-2000) and cholesterol to prepare liposomes in the form of unilamellar vesicles.
[0081] In detail, the SA-V6-NH 2 Dissolve in methanol, and dissolve DPPC, DSPE-PEG and cholesterol in chloroform. After mixing the methanol and chloroform solutions in a round bottom flask, a lipid thin layer was formed on the inner wall of the flask by evaporating the solvent at room temperature using a rotary evaporator.
[0082] Next, the liquid thin layer was hydrated by adding physiological saline in which 200 mM calcein was dissolved to the flask at room temperature. Calcein is ...
Embodiment 2
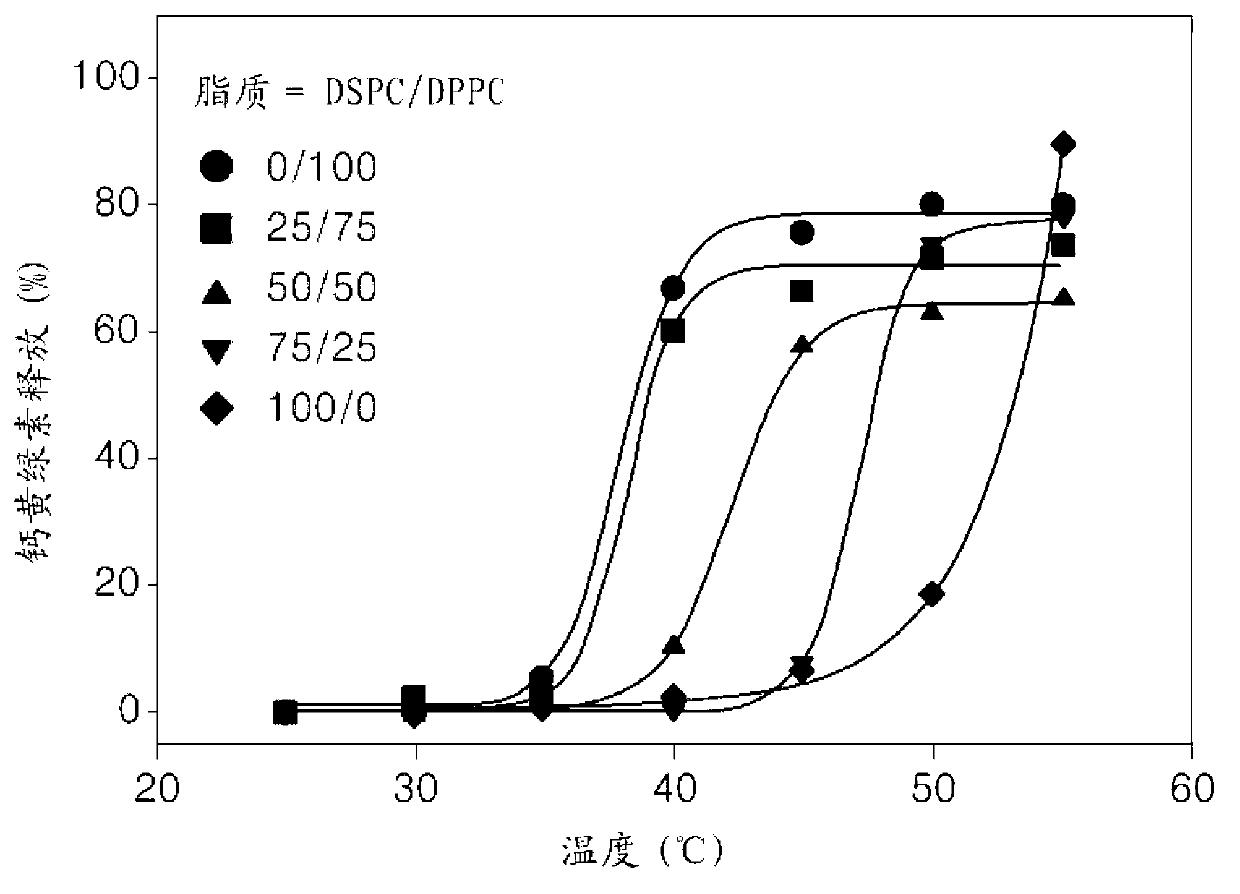
[0089] Liposomes were prepared according to the same method used in Example 1, except that 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) was added to DPPC as the main lipid component, and DPPC / DSPC was mixed in molar ratios of 0 / 100, 25 / 75, 50 / 50, 75 / 25, 100 / 0 and used mainly lipid molecules. Then, the heat sensitivity of the liposomes was assessed. The prepared liposomes had a similar mean diameter and distribution to the liposomes prepared in Example 1.
[0090] figure 2 It is to show that calcein passes through according to embodiment 2 with the molar ratio of about 0.55:about 55 (0 / 100,25 / 75,50 / 50,75 / 25,100 / 0 (by molar ratio)):about 2:about 20 Use SA-V6-NH 2 , graphs of temperature release profiles in liposomes prepared from major lipid molecules (DPPC / DSPC), DSPE-PEG and cholesterol. Such as figure 2 As shown in , the onset temperature of calcein release increases as the amount of DSPC of the main lipid molecule increases. When DSPC 100 was used as the main l...
Embodiment 3
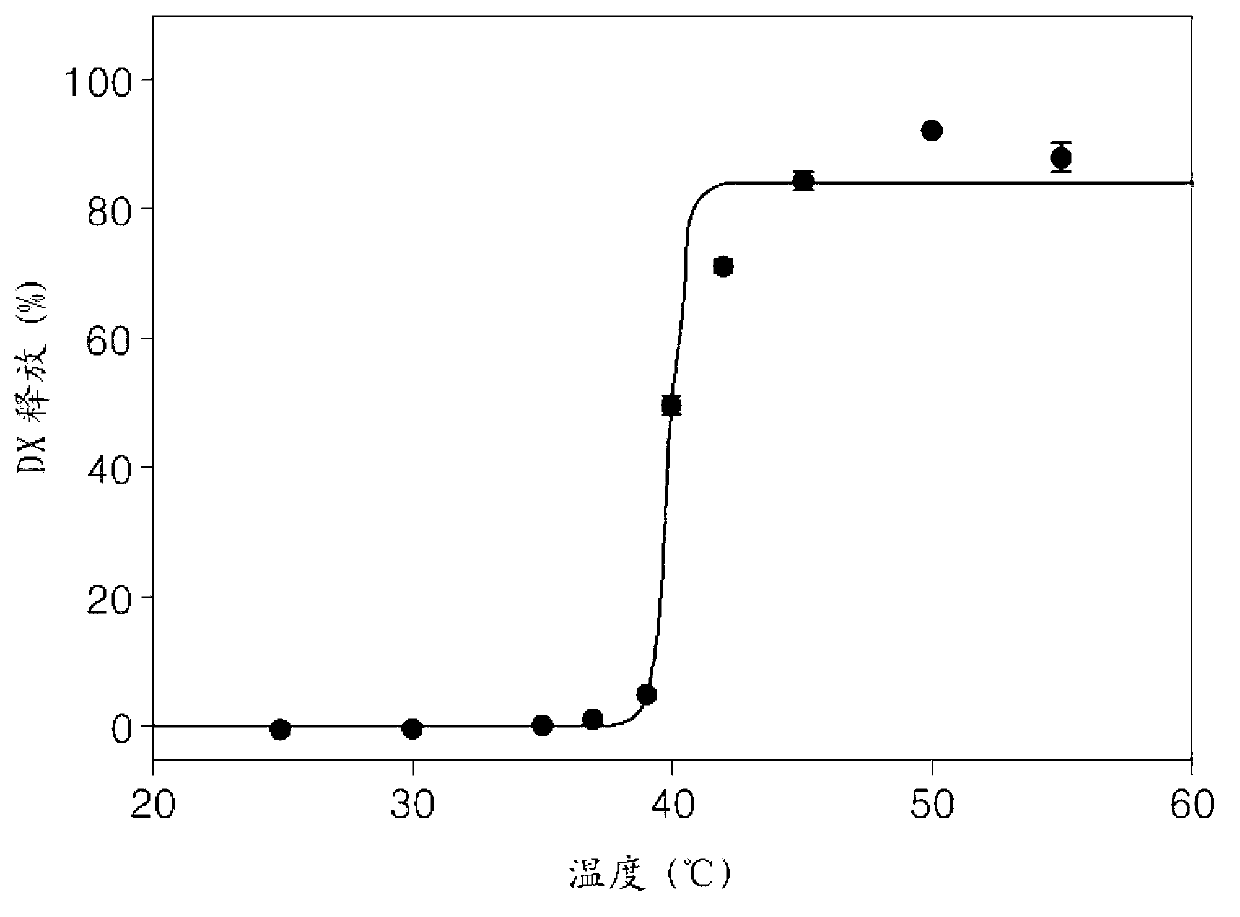
[0091] Example 3: Preparation and Thermosensitivity Measurement of Doxorubicin-Containing Liposomes Using the Ammonium Sulfate Gradient Method
[0092] DSPC and DPPC were used as the main lipid components at a mixing ratio of 25:75 and SA-V3-NH at a molar ratio of 0.55:55:2:20 2 , DSPC+DPPC, DSPE-PEG, and cholesterol, and use the ammonium sulfate gradient method (J. Control. Release 2009, 139, 73-80) to prepare doxorubicin-encapsulated unilamellar vesicular liposomes.
[0093] In detail, stearoyl-VPGVG VPGVG VPGVG-NH 2 (SEQ NO:7, hereinafter referred to as "SA-V3-NH 2 ”) was dissolved in methanol, and DSPC, DPPC, DSPE-PEG and cholesterol were dissolved in chloroform. After mixing the methanol and chloroform solutions in a round bottom flask, evaporate the solvent in the flask by using a rotary evaporator at room temperature A thin layer of lipids forms on the inner wall.
[0094] Next, the lipid lamella was hydrated by adding a 250 mM ammonium sulfate solution to the flask ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase transition temperature | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com