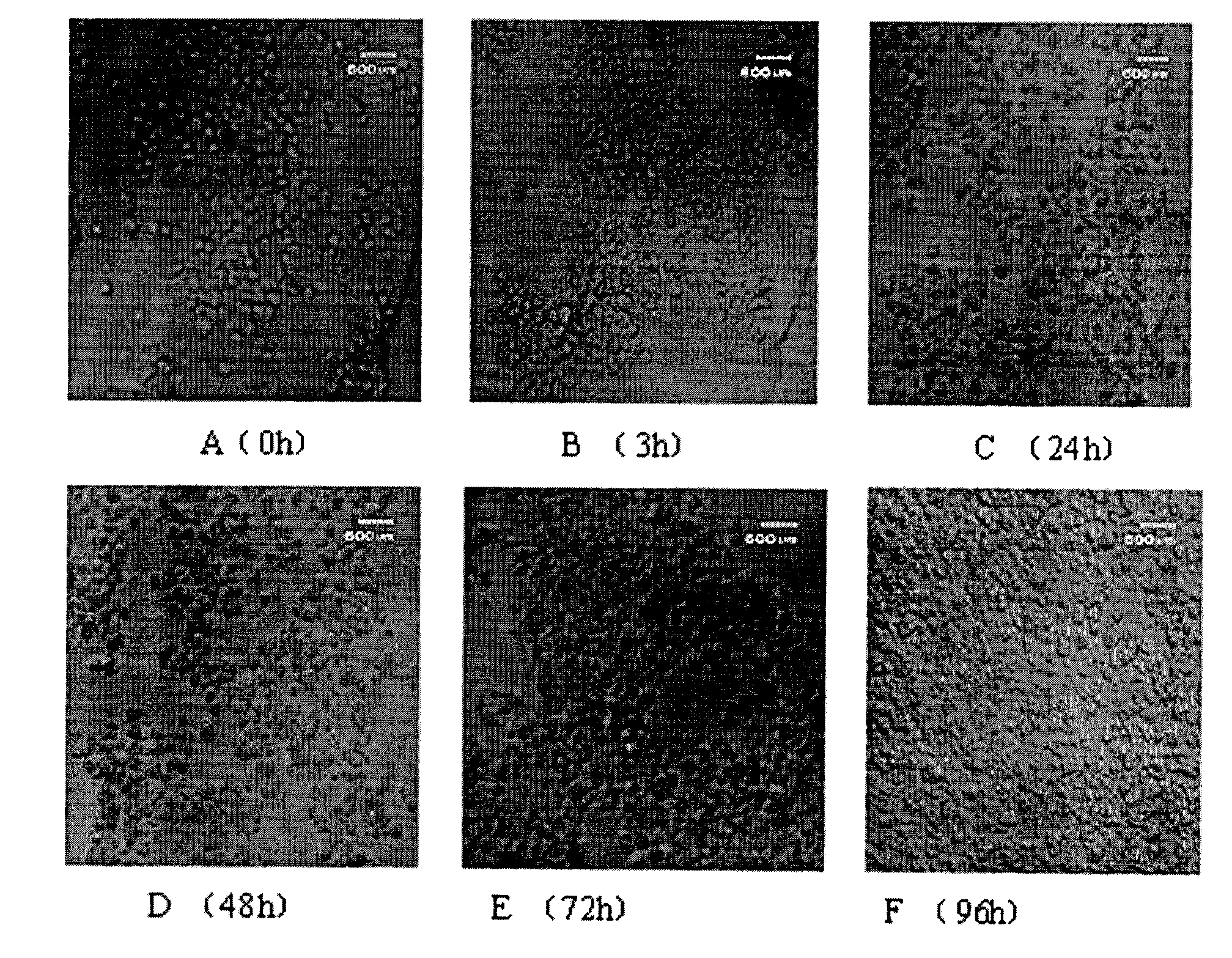
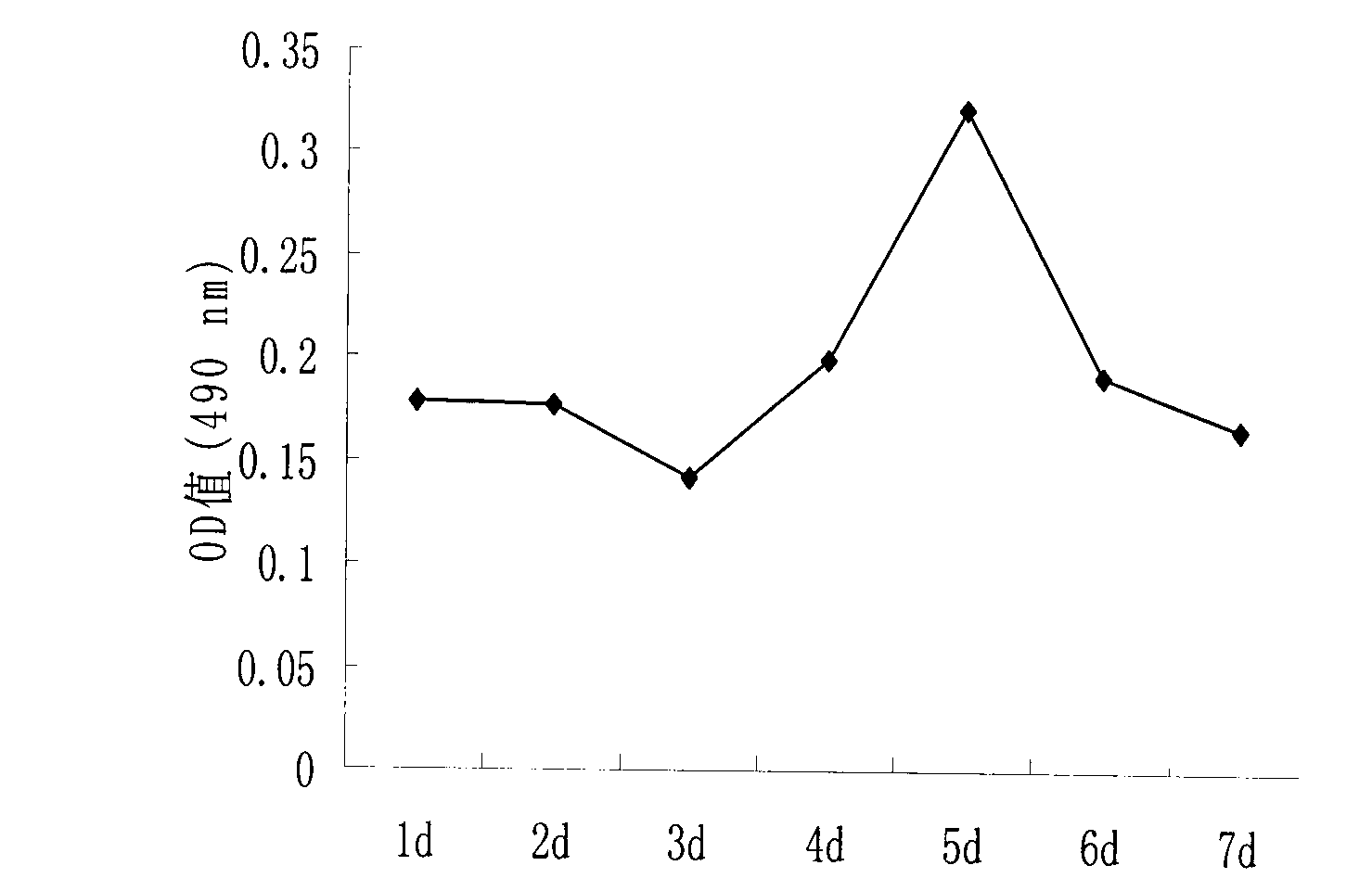
Goose primary hepatocyte isolation culture method
A primary hepatocyte, isolation and culture technology, applied to animal cells, vertebrate cells, artificial cell constructs, etc. The effect of uniform flow and less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The isolation and culture method of goose primary hepatocytes, its operating steps are:
[0039] (1) Inject pentobarbital sodium intraperitoneally into geese fasting for 12 hours. The injection volume is 30 mg / kg body weight and 100 IU / kg body weight of heparin sodium. After it is anesthetized, the supine position is fixed and the abdomen is sterilized;
[0040] (2) Cut the abdominal cavity along the midline of the abdomen of the goose, quickly take out the complete liver, and wash the surface of the liver with 40°C normal saline;
[0041] (3) The washed liver was perfused with preperfusate at 40°C until the liver turned pale yellow;
[0042] (4) Replace with 40°C cleaning solution to wash out the preperfusate in the liver;
[0043] (5) Repeated perfusion with 0.05% enzyme perfusate at 40°C until the subhepatic capsule showed a turtle-like fissure;
[0044] (6) Put the liver into a glass petri dish, tear off the capsule, and cut the liver into pieces;
[0045] (7) Po...
Embodiment 2
[0059] 1. Experimental method
[0060] Isolation and Culture of Goose Primary Hepatocytes
[0061] (1) Disinfection anesthesia: intraperitoneally inject pentobarbital sodium (30 mg / kg body weight) and heparin sodium (100 IU / kg body weight) into the geese fasting for 12 hours, wait for anesthesia (15-30 min), fix the supine position, and the abdomen is covered with skin disinfection;
[0062] (2) Liver extraction: cut the abdominal cavity along the midline of the abdomen of the goose, quickly remove the complete liver, and wash the surface of the liver with 40°C normal saline;
[0063] (3) Lavage: The washed liver is rapidly perfused with 40°C preperfusate at a rate of about 30ml / min until the liver turns light yellow; then replaced with 40°C wash solution to wash out the preperfusate in the liver;
[0064] (4) Digestion: transfer the liver to another sterile plate, replace with enzyme perfusate (preheated at 40°C) and repeatedly perfuse and digest for 10-30min at a perfusion...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com