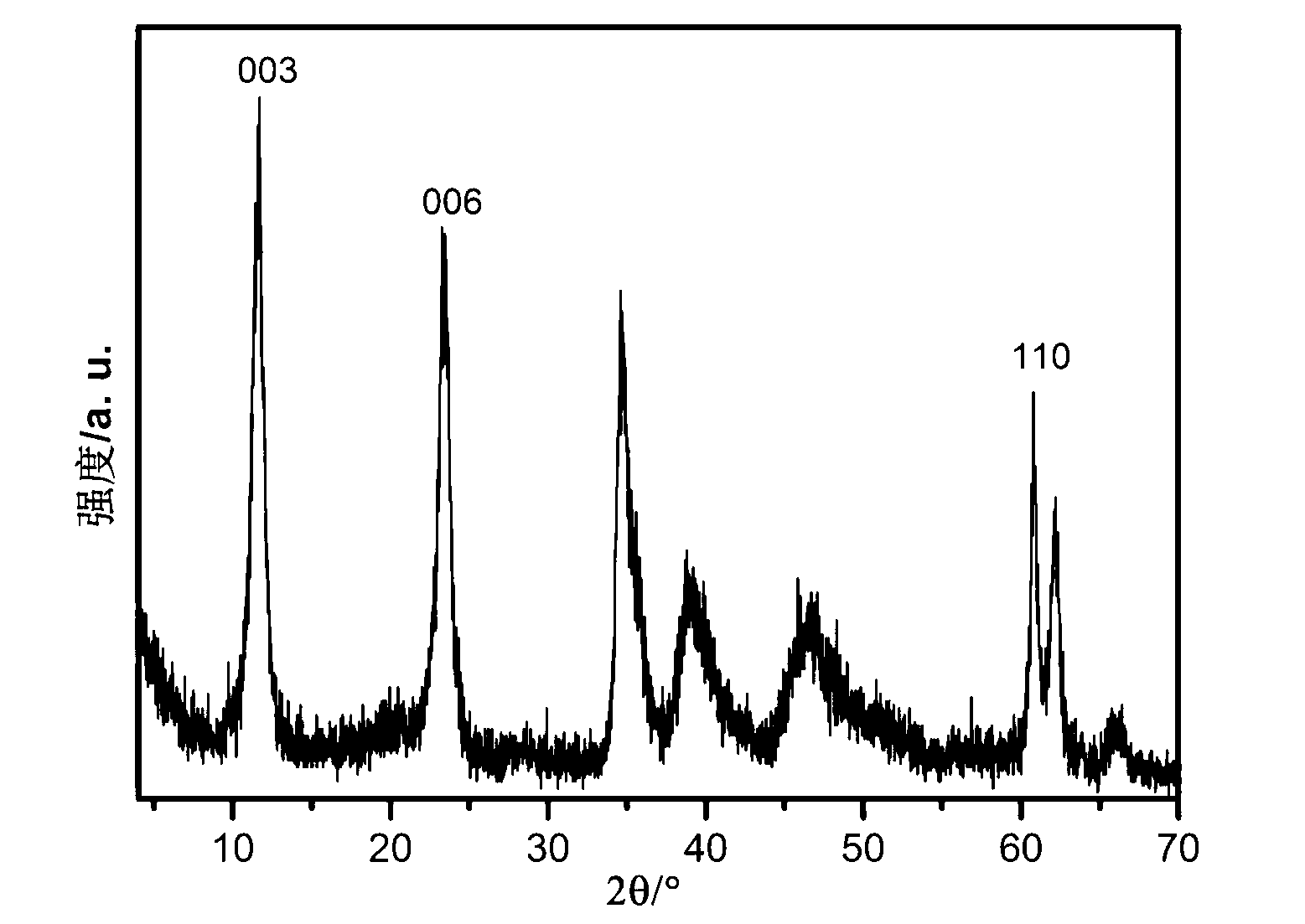
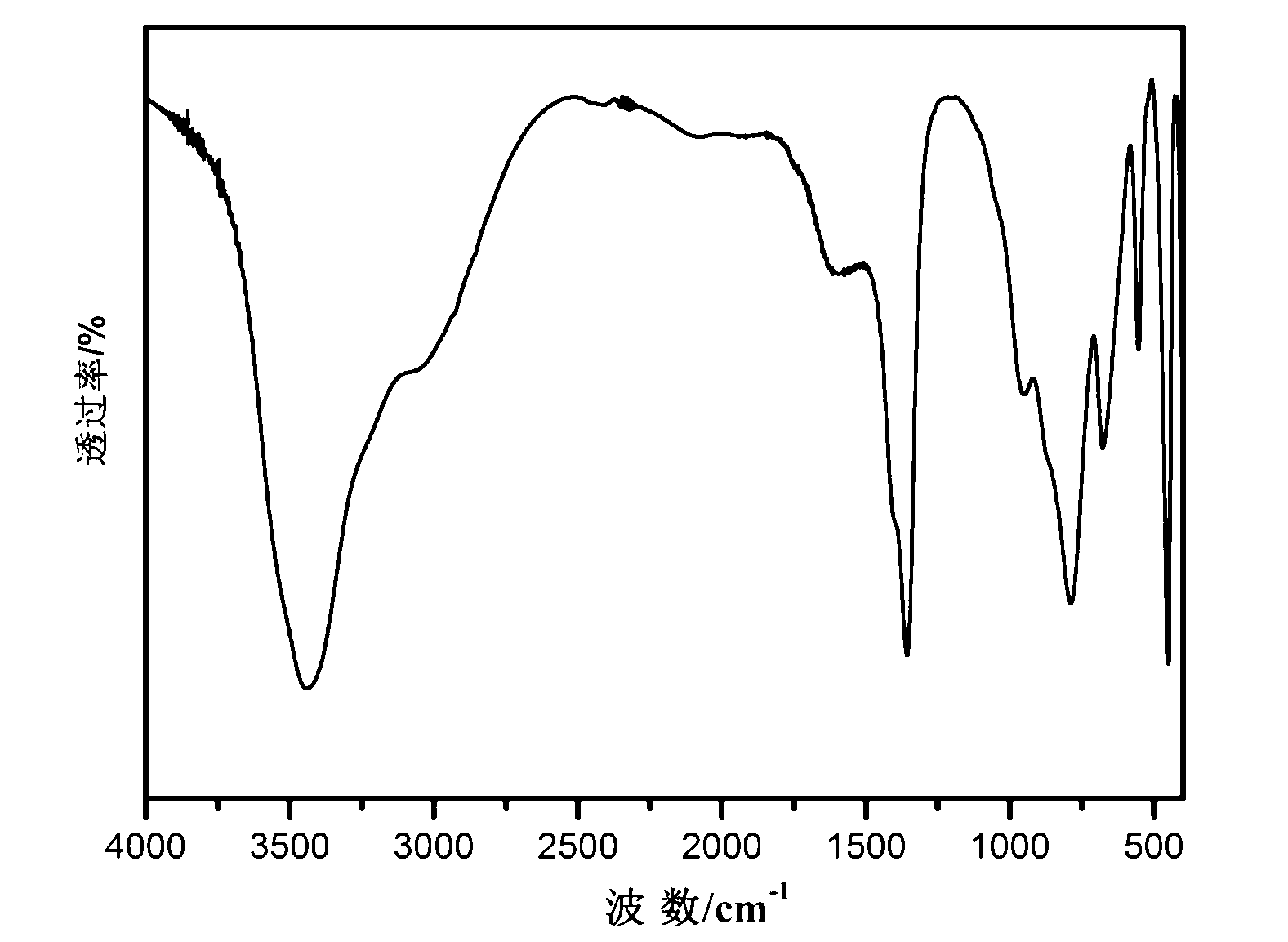
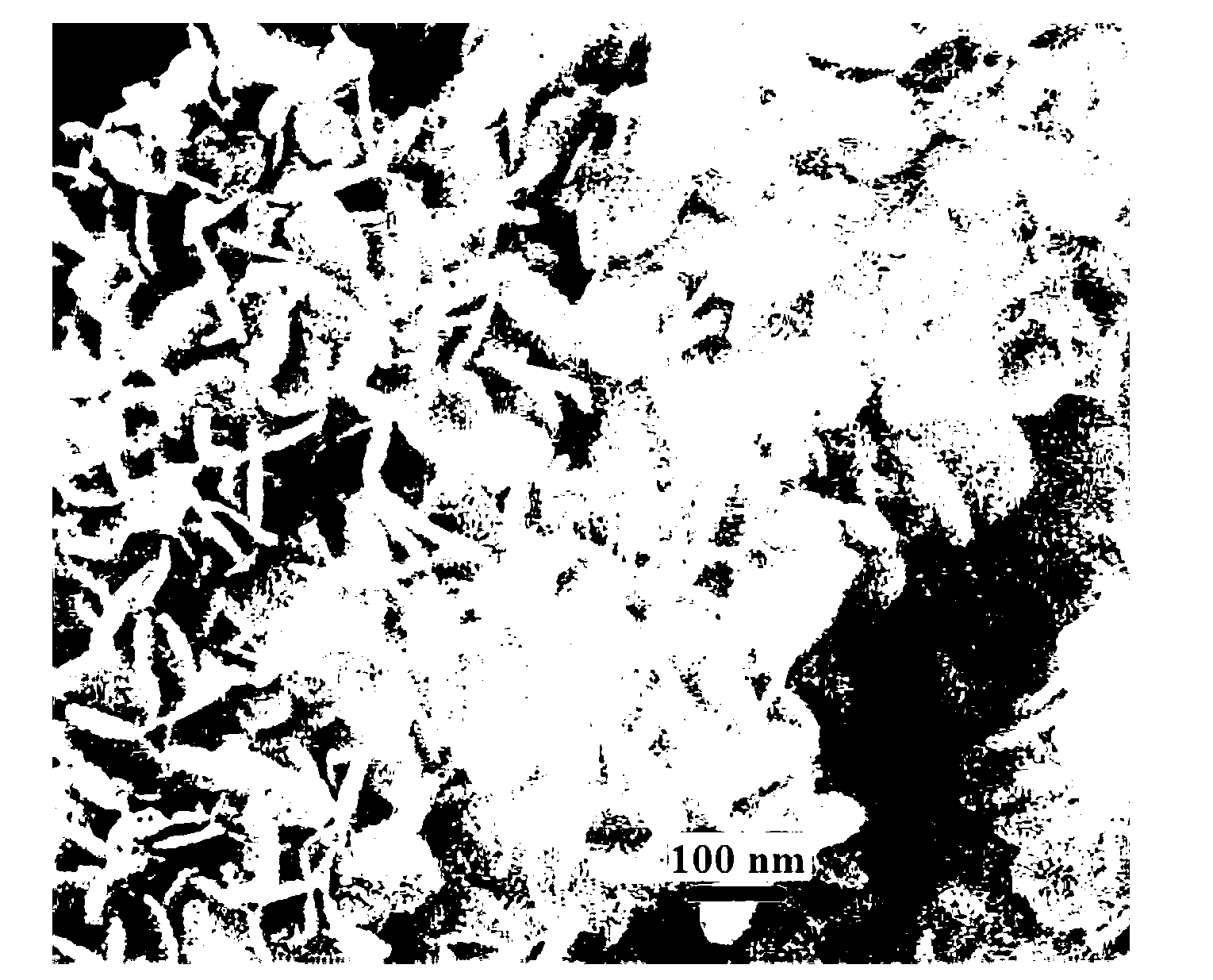
Nano-size layered double hydroxide and step-by-step precipitation preparation method thereof
A layered composite and hydroxide technology, applied in chemical instruments and methods, nickel compounds, titanium compounds, etc., can solve problems such as application performance limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Step A: take by weighing 18.75g Al(NO 3 ) 3 .9H 2 O was added to 100.25 g of deionized water to make a solution with a concentration of about 0.5 mol / L.
[0030] Step B: take by weighing 6gNaOH and join in 75g deionized water and be mixed with alkali solution, this alkali solution is mixed with the Al(NO 3 ) 3 The solution was mixed to form aluminum hydroxide precipitate, and then left to age for 10 minutes.
[0031] Step C: take by weighing 1.92g Mg(NO 3 ) 2 .6H 2 O is added in the aluminum hydroxide precipitation slurry that 10g step B prepares, mix well, make Mg(NO 3 ) 2 .6H 2 O is completely dissolved.
[0032] Step D: Weigh 2.4g urea and 0.54g Na 2 CO 3 Add to 40ml of deionized water to prepare an alkaline solution.
[0033] Step E: Add the alkali solution prepared in step D directly to the mixture prepared in step C under stirring at 80°C, and then continue to stir and react at 100°C for 24 hours. After the reaction, cool the slurry to room temperatur...
Embodiment 2
[0035] Steps A and B are the same as in Example 1.
[0036] Step C: take by weighing 2.56g Mg(NO 3 ) 2 .6H 2 O is added in the aluminum hydroxide precipitation slurry that 10g step B prepares, mix well, make Mg(NO 3 ) 2 .6H 2 O is completely dissolved.
[0037] Step D: Weigh 3.3g urea and 0.54g Na 2 CO 3 Add to 40ml of deionized water to prepare an alkaline solution.
[0038] Step E: Add the alkali solution prepared in step D directly to the mixture prepared in step C at 90°C with stirring, then continue to stir and react at 100°C for 20 hours, cool the slurry to room temperature after the reaction, and remove the precipitate Wash by centrifugation until the pH value is less than 8, and dry the sample in an oven at 100°C for 12 hours to obtain the LDH product. Elemental analysis shows that the chemical composition formula of the product is: Mg 0.8 Al 0.2 (OH) 2 (CO 3 ) 0.1 0.63H 2 O, BET analysis shows that its specific surface area is 177m 2 / g.
Embodiment 3
[0040] Steps A and B are the same as in Example 1.
[0041] Step C: take by weighing 0.641g Mg(NO 3 ) 2 .6H 2 O and 0.727g Ni(NO 3 ) 2 .6H 2 O is added in the aluminum hydroxide precipitation slurry that 10g step B prepares, mix well, make Mg(NO 3 ) 2 .6H 2 O and Ni(NO 3 ) 2 .6H 2 O is completely dissolved.
[0042] Step D: Weigh 1.6g urea and 0.2g Na 2 CO 3 Add to 40ml of deionized water to prepare an alkaline solution.
[0043] Step E: Add the alkali solution prepared in step D directly to the mixture prepared in step C at 95°C with stirring, then continue to stir and react at 100°C for 10 hours, cool the slurry to room temperature after the reaction, and remove the precipitate Wash by centrifugation until the pH value is less than 8, and dry the sample in an oven at 100°C for 12 hours to obtain the LDH product. Elemental analysis shows that the chemical composition formula of the product is: Mg 0.33 Ni 0.33 Al 0.33 (OH) 2 (CO 3 ) 0.165 0.63H 2 O, BET a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com