Impurity removal method for traditional Chinese medicine injection
A technology for injections and traditional Chinese medicines, which is used in pharmaceutical formulations, drug delivery, and medical preparations containing active ingredients, and can solve problems such as irritation to the human body and unsatisfactory effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Embodiment 1 Chinese medicine injection intermediate liquid dosing preparation method
[0107] a. Preparation of Astragalus extract: Take Astragalus decoction pieces, add 8 times the amount of water to soak for 1 hour, heat and decoct for extraction for 3 hours, filter, and use the filtrate for later use; add 6 times of water to the dregs, decoct and extract twice, each time for 2 hours, filter. Combine the filtrates three times, concentrate to a relative density of 1.05-1.20 (50°C), cool to room temperature, add ethanol until the alcohol content reaches 80v / v, and refrigerate for 12h. The supernatant was poured out, and the dregs were centrifugally filtered. Combine the supernatant and filtrate, recover ethanol under reduced pressure and concentrate to a relative density of 1.05-1.20 (50°C). Inject the concentrated solution into the treated D101 macroporous adsorption resin, wash it with deionized water until it is colorless, and discard the water; then elute with 30...
Embodiment 2
[0110] Embodiment 2 intermediate impurity removal method
[0111] According to the method described in Example 1, the intermediate formulation was prepared.
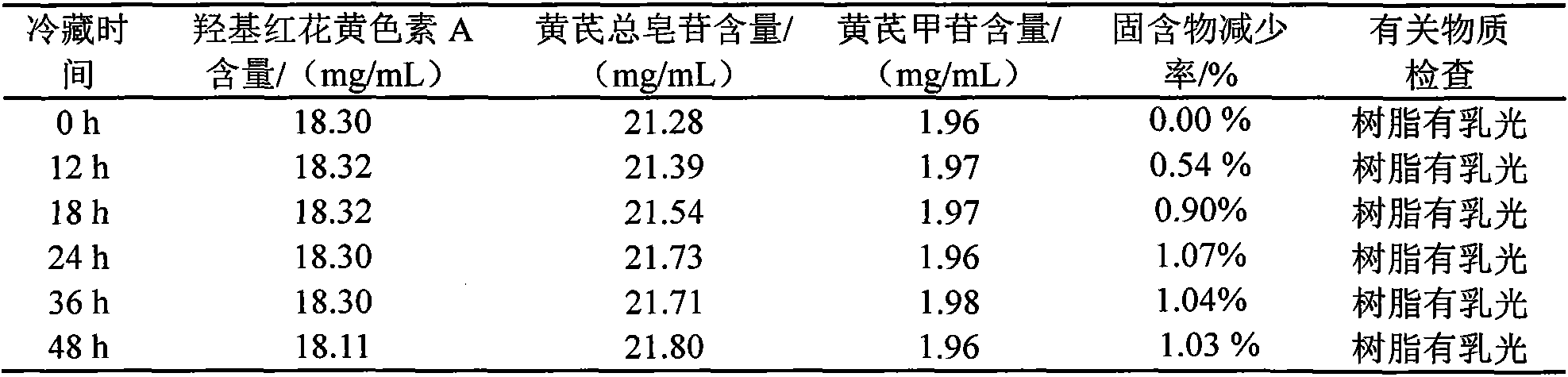
[0112] Take the intermediate solution and refrigerate for 24 hours, filter, and take the supernatant I; centrifuge the supernatant I obtained in step 1 at a rate of 5000r / min for 15 minutes, and take the supernatant II; the supernatant obtained in step 2 Add 0.3% activated carbon by weight to II for adsorption, the adsorption time is 30min, and the adsorption temperature is 80°C. During the adsorption process, potassium hydroxide (KOH) is used to adjust the pH value to keep the supernatant pH value unchanged. After adsorption, filter and collect the filtrate.
Embodiment 3
[0113] Embodiment 3 intermediate impurity removal method
[0114] According to the method described in Example 1, the intermediate formulation was prepared.
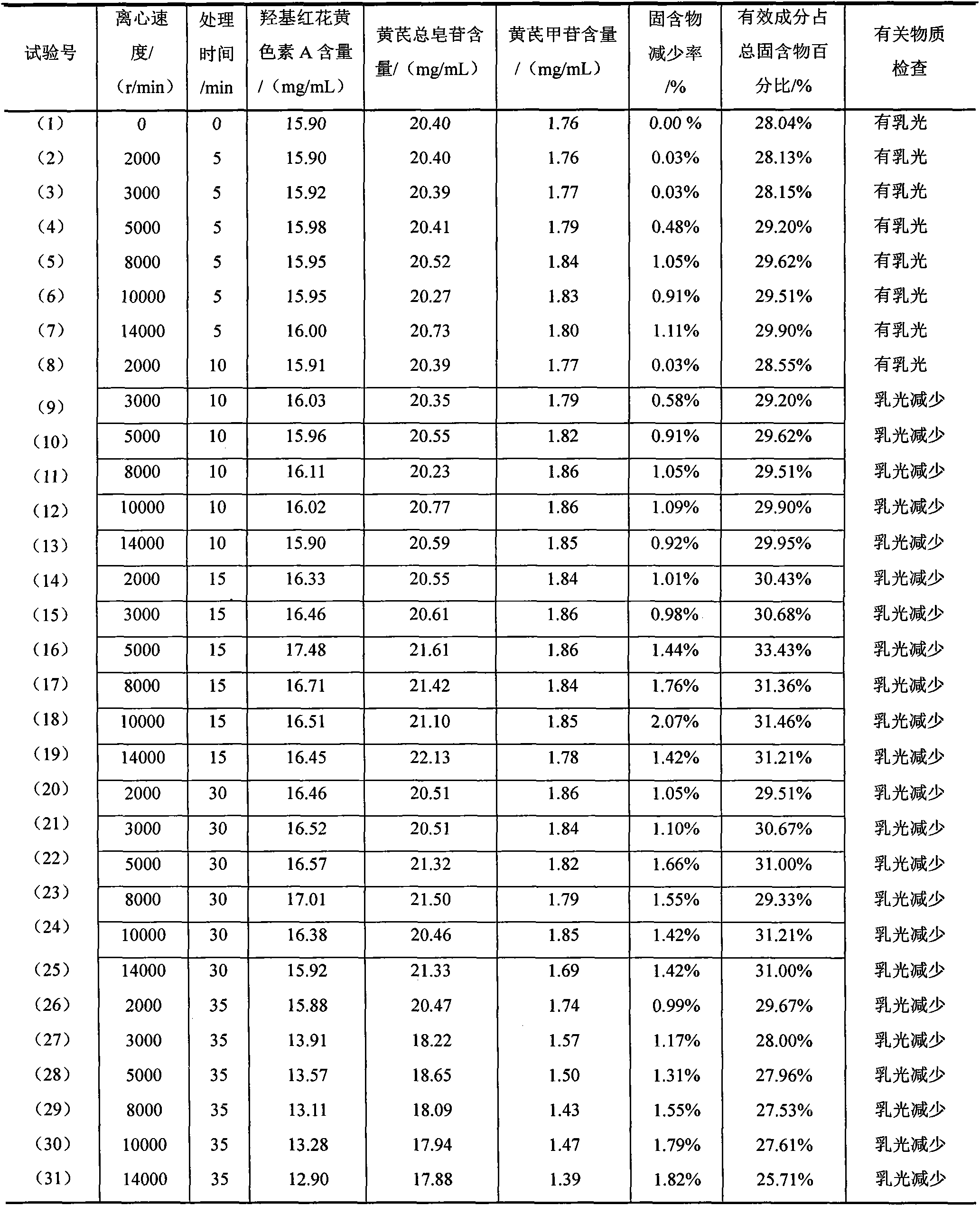
[0115] Take the intermediate solution and refrigerate for 18 hours, filter, and take the supernatant I; centrifuge the supernatant I obtained in step 1 at a rate of 8000r / min for 10 minutes, and take the supernatant II; the supernatant obtained in step 2 Add 0.5% activated carbon by weight to II for adsorption, the adsorption time is 45min, and the adsorption temperature is 90°C. During the adsorption process, the pH value is adjusted with meglumine solution, so that the pH value of the supernatant remains unchanged. After adsorption, filter and collect the filtrate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com