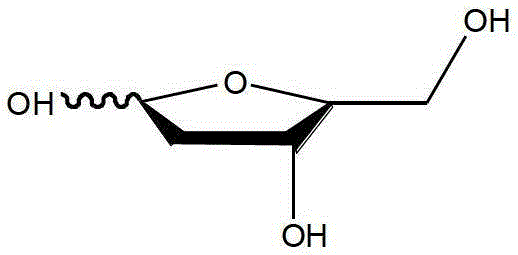
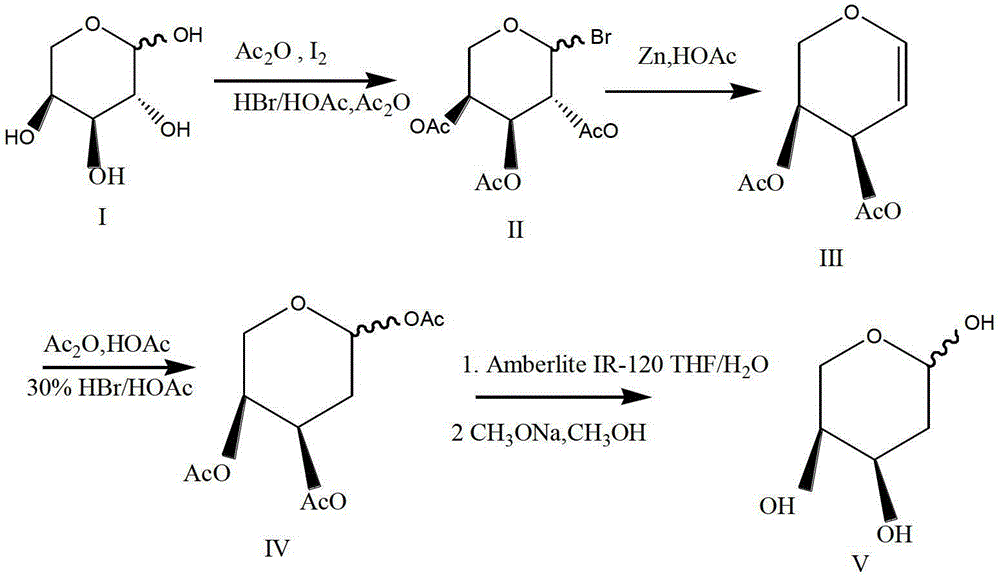
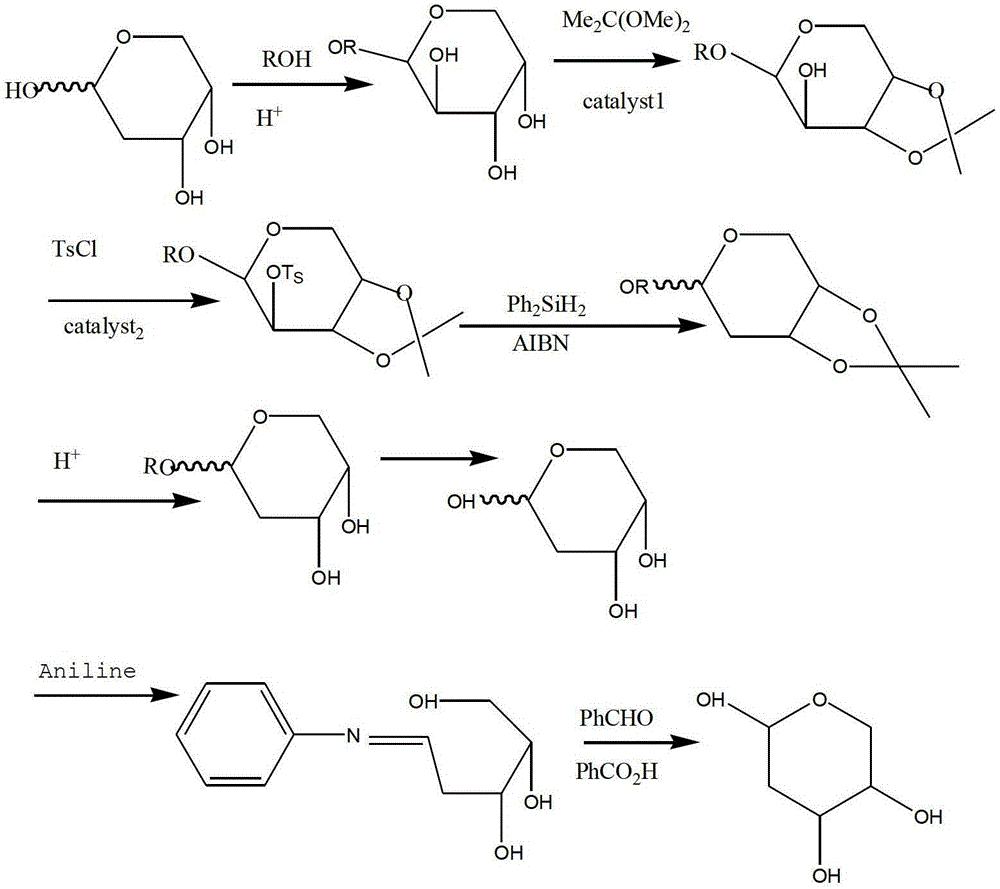
The preparation method of 2-deoxy-l-ribofuranose
A technology of ribofuranose and tetrahydrofuran, which is applied in the field of medicine, can solve the problems of flammable raw materials, long steps, expensive and difficult to obtain raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] (1) Methylation: Put 100g of L-arabinose and 800mL of methanol with a concentration of 95% into the reaction kettle, stir and dissolve, add HCl methanol solution dropwise, the concentration of HCl in it is 0.01mol / l, and react at room temperature for 5 hours. After the reaction is completed, add sodium carbonate to neutralize, filter, and put the solution into the next step after the solution is spin-dried;
[0060] (2) Protection of hydroxyl groups at positions 3 and 5: Put the product from the previous step into the reactor, add 400 mL of tetrahydrofuran, 110 g of benzaldehyde dimethyl acetal and 5 g of p-toluenesulfonic acid as a catalyst, and react at 50°C. After the reaction is completed, the remaining benzaldehyde dimethyl acetal and tetrahydrofuran are removed by distillation, toluene is added and washed with sodium bicarbonate solution to neutrality, the solvent is removed by distillation under reduced pressure, crystallized with methanol, and put into the next s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com