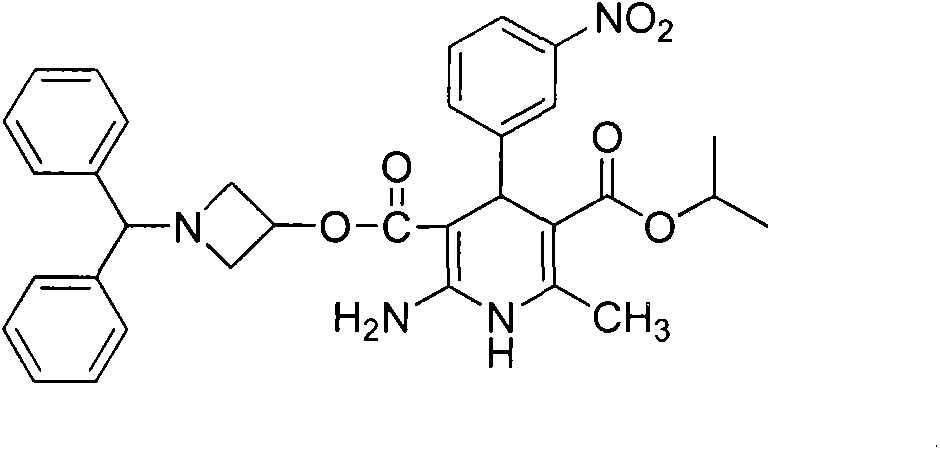
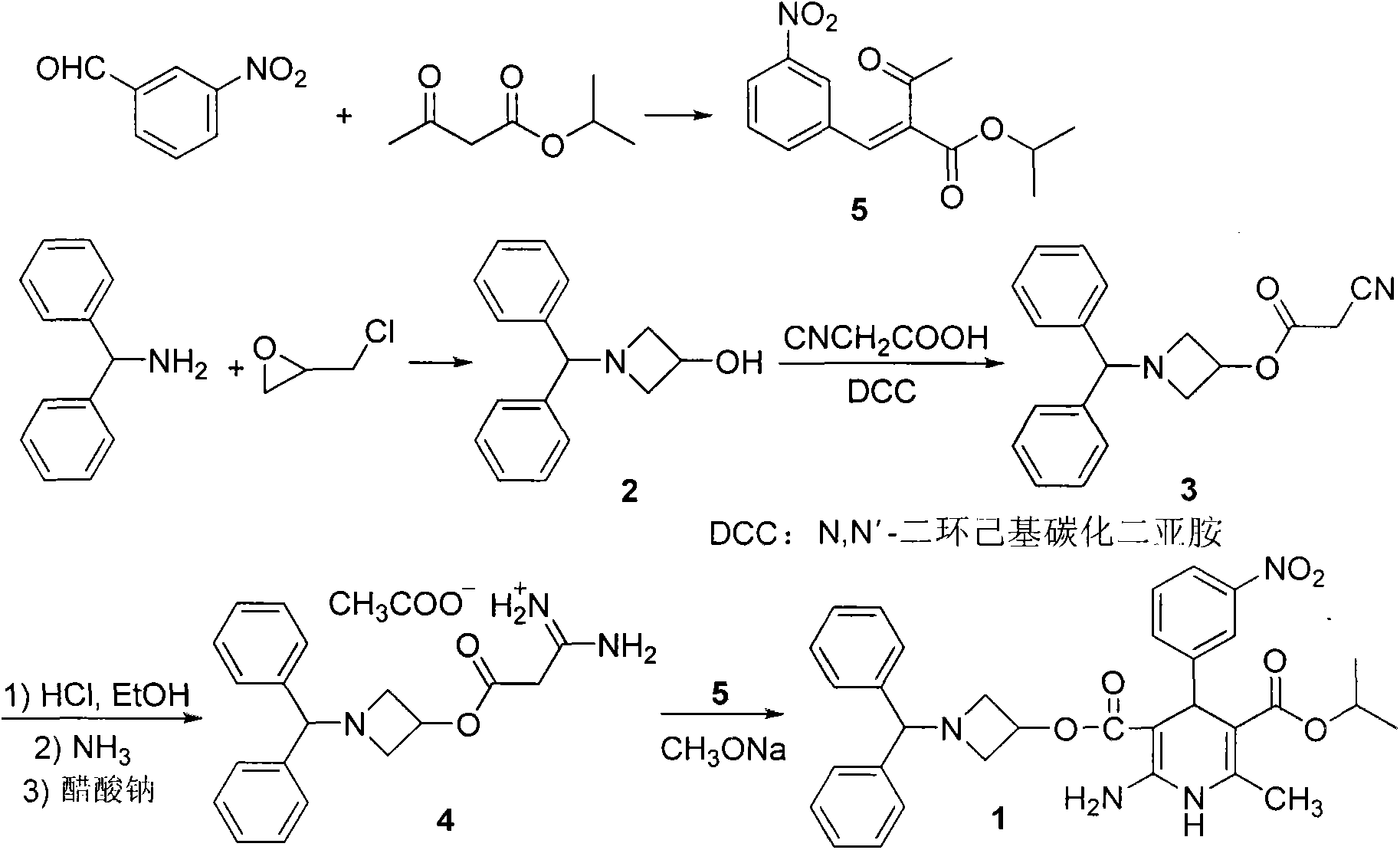
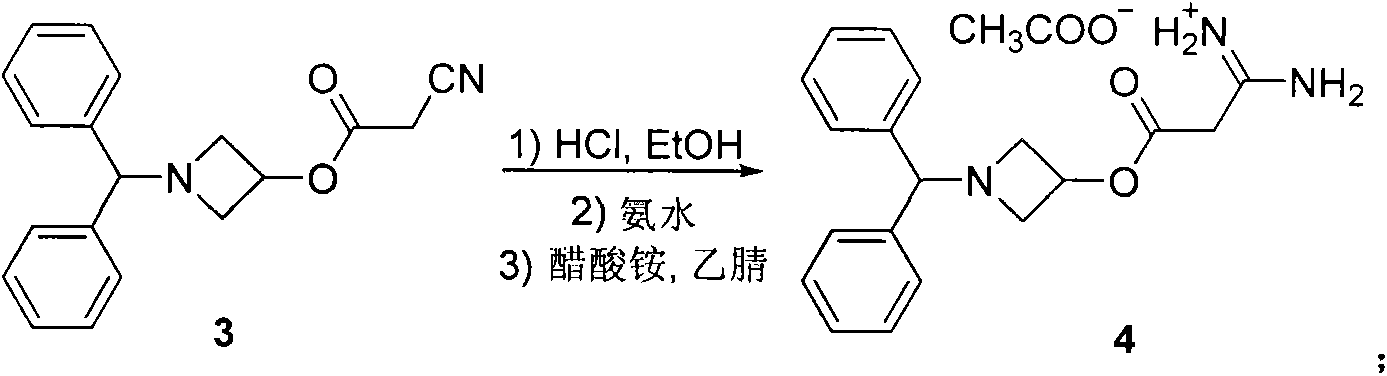
Preparation method for azelnidipine
A kind of technology of azelaine and compound, which is applied in the field of preparation of azeldipine, can solve the problems of increased cost, difficult handling, difficult industrialized production, etc., and achieves the effect of reducing the requirement of anhydrous degree and being easy to operate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Preparation of Azeldipine
[0029] Add 50g of compound 3, 1500mL of dichloromethane, and 16.64mL of absolute ethanol into a 5L three-neck flask. Under mechanical stirring, pass HCl gas below -5°C to saturation. After saturation, keep the reaction at -5°C for 24h. Protected from light and protected by nitrogen, slowly add the above reaction system to 1665ml of ammonia water with a concentration of 2.5-3.0% under the control of 0-5°C. After the addition is complete, stir for 0.5h, let stand for 0.5h, and separate the liquids. The dichloromethane layer was washed once with 2000mL saturated brine, left to stand for 1.0h, separated, and the dichloromethane layer was drained under reduced pressure to obtain a white solid, which was not dried. It was directly added to 2000mL acetonitrile, slowly heated to dissolve, and added 11.7g of ammonium acetate, temperature controlled at 55°C-60°C, reacted for 2h under mechanical stirring. The temperature was lowered, the ...
Embodiment 2
[0031] Embodiment 2; Preparation of Azeldipine
[0032] Add 50g of compound 3, 1500mL of dichloromethane, and 16.64mL of absolute ethanol into a 5L three-necked flask. Under mechanical stirring, pass HCl gas below -5°C to saturation. After saturation, keep it at -6°C to -8°C for 24 hours. Under the condition of controlling 0-5°C, slowly add the above reaction system into ammonia water with a concentration of 2.5-3.0%, adjust the pH to 7.8-8.5, after the addition is completed, stir for 0.5h, stand still for 0.5h, and separate the liquids. The dichloromethane layer was washed once with 2000mL saturated brine, left to stand for 1.0h, separated, and the dichloromethane layer was drained under reduced pressure to obtain a white solid, which was not dried. It was directly added to 2000mL acetonitrile, slowly heated to dissolve, and added 11.7g of ammonium acetate, temperature controlled at 55°C-60°C, reacted for 2h under mechanical stirring. After cooling down, the solid was precip...
Embodiment 3
[0034] Embodiment 3: the preparation of amidinate 4
[0035] Add 50g of compound 3, 1500mL of dichloromethane, and 16.64mL of absolute ethanol into a 5L three-neck flask. Under mechanical stirring, pass HCl gas below -5°C to saturation. After saturation, keep it at -7°C to -9°C for 24 hours. Slowly add the above reaction system to ammonia water with a concentration of 2.5-3.0% under controlled conditions of 0-5°C, adjust the pH to 8.5-9.5, after the addition is complete, stir for 0.5h, let stand for 0.5h, and separate the liquids. The dichloromethane layer was washed once with 2000mL saturated brine, left to stand for 1.0h, separated, and the dichloromethane layer was drained under reduced pressure to obtain a white solid, which was not dried. It was directly added to 2000mL acetonitrile, slowly heated to dissolve, and added 11.7g of ammonium acetate, temperature controlled at 55°C-60°C, reacted for 2h under mechanical stirring. After cooling down, the solid was precipitated,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com