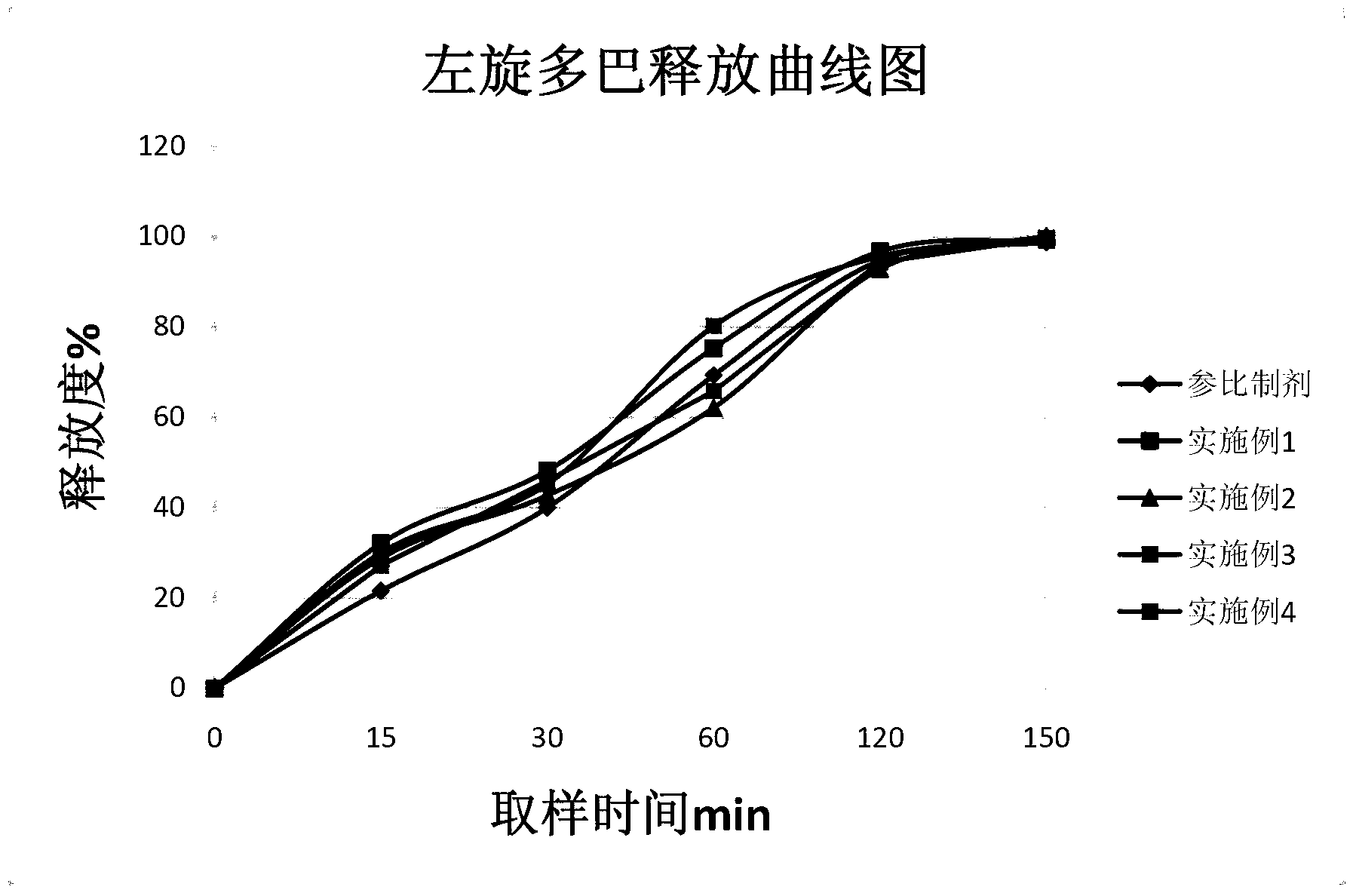
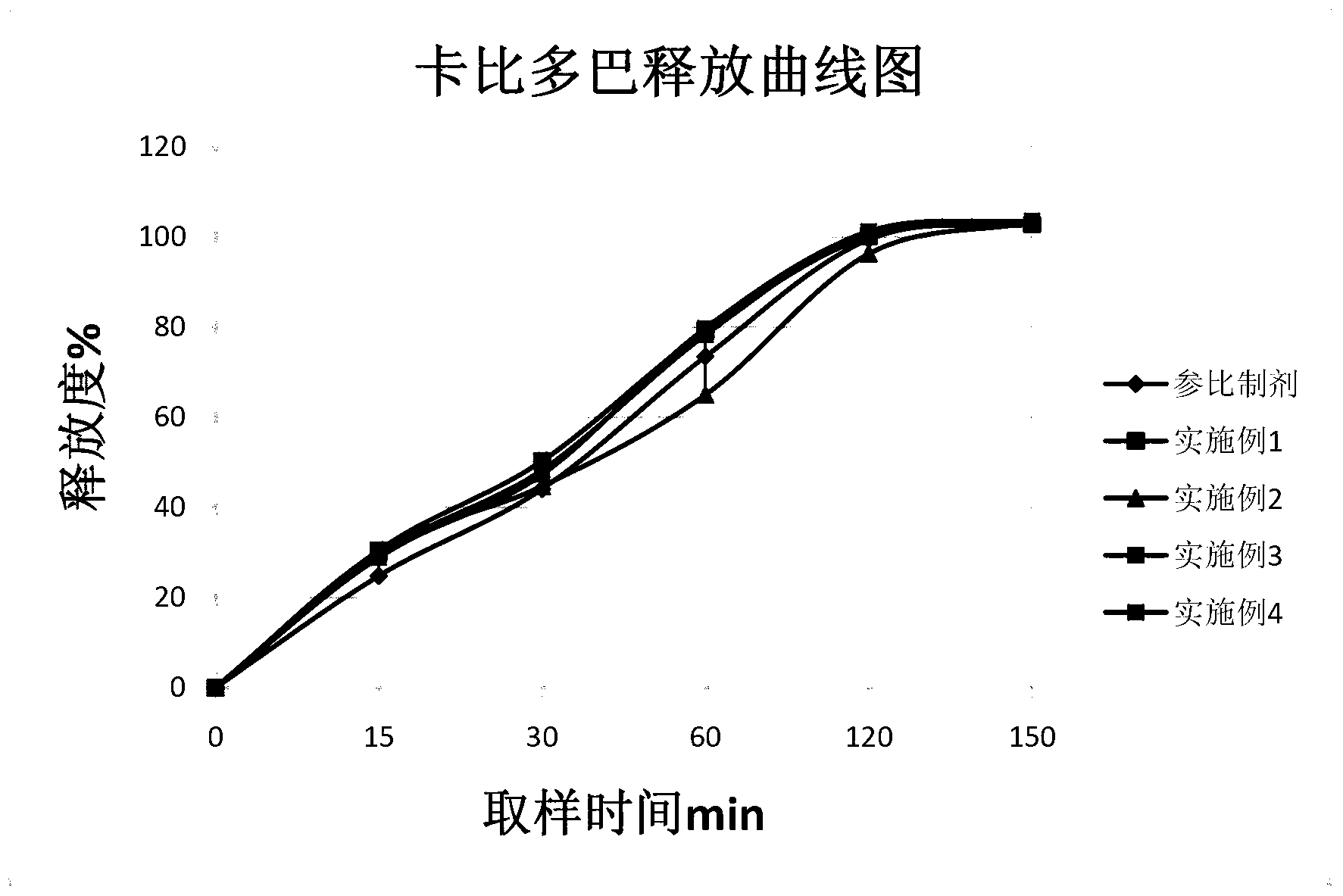
Carbidopa-levodopa controlled release tablet and preparation method of tablet
A slow-release tablet and levodopa technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of small total weight of tablets, restrictions on industrial production, and production processes Problems such as development difficulty, to achieve the effect of easy industrial production, improve safety and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
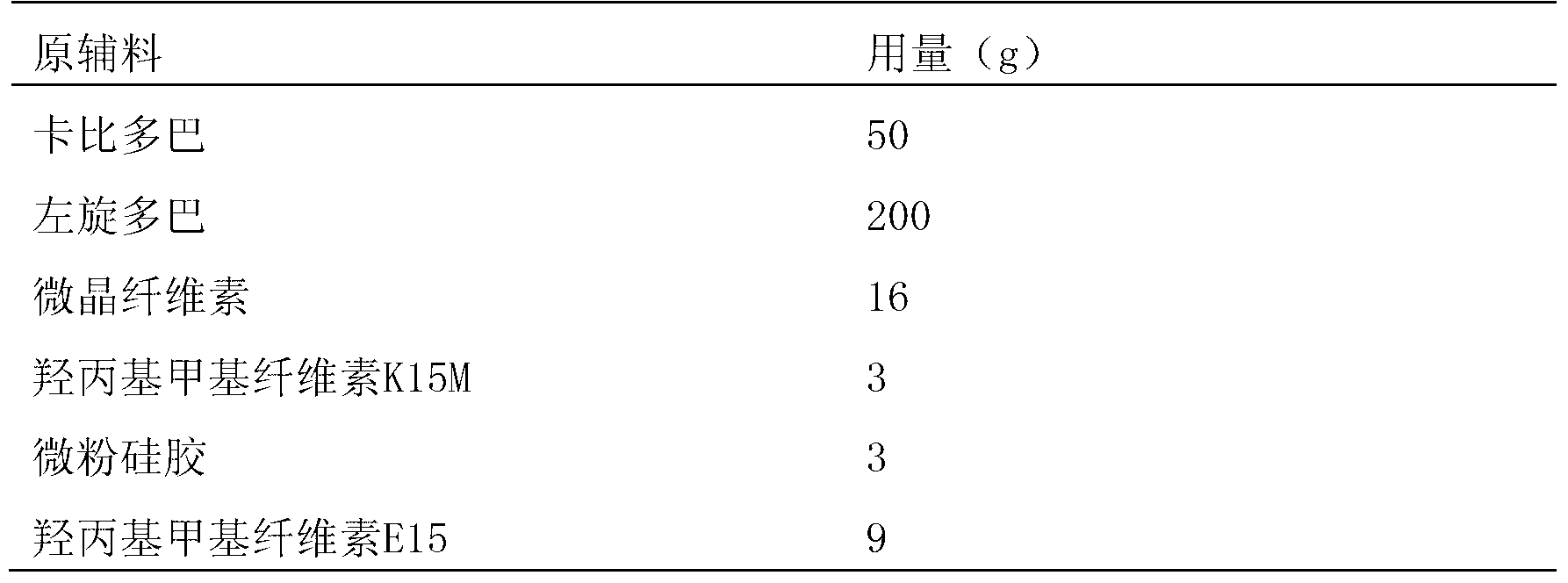
Embodiment 1
[0024] Prescription composition (dosage per 1000 tablets, unit: g)
[0025]
[0026]
[0027] Weigh hydroxypropyl methylcellulose E15 and Opadry according to the prescription amount, add 95% ethanol and water to dissolve and use it as a coating solution for later use; carbidopa, levodopa, microcrystalline cellulose, hydroxypropyl Mix methylcellulose K15M and micropowder silica gel evenly, add the above-mentioned coating solution for fluidized bed granulation, dry, add additional magnesium stearate, mix evenly, and press into tablets with a conventional tablet machine.
Embodiment 2
[0029] Prescription composition (dosage per 1000 tablets, unit: g)
[0030]
[0031] Weigh hydroxypropyl methylcellulose E15 and Opadry according to the prescription amount, add 95% ethanol and water to dissolve and use it as a coating solution for later use; carbidopa, levodopa, microcrystalline cellulose, hydroxypropyl Mix methylcellulose K4M and micropowder silica gel evenly, add the above-mentioned coating solution for fluidized bed granulation, dry, add additional magnesium stearate, mix evenly, and press into tablets with a conventional tablet machine.
Embodiment 3
[0033] Prescription composition (dosage per 1000 tablets, unit: g)
[0034]
[0035]
[0036] Weigh hydroxypropyl methylcellulose E15 and Opadry according to the prescription amount, add 95% ethanol and water to dissolve and use it as a coating solution for later use; carbidopa, levodopa, microcrystalline cellulose, hydroxypropyl Mix methylcellulose K15M and micropowder silica gel evenly, add the above-mentioned coating solution for fluidized bed granulation, dry, add additional magnesium stearate, mix evenly, and press into tablets with a conventional tablet machine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com