Mesogen-jacketed polymer monomers of norbornene imide system and polymers of polymer monomers
A technology of alkenyl imide and chitosan, which is applied in the field of chitosan polymer of norbornene imide system and its preparation, which can solve the problem of long synthesis route of MJLCP monomer, non-functionality of the main chain, and production conditions Harshness and other problems, to achieve the effect of simplifying the solvent treatment process, simplifying the synthesis route, and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
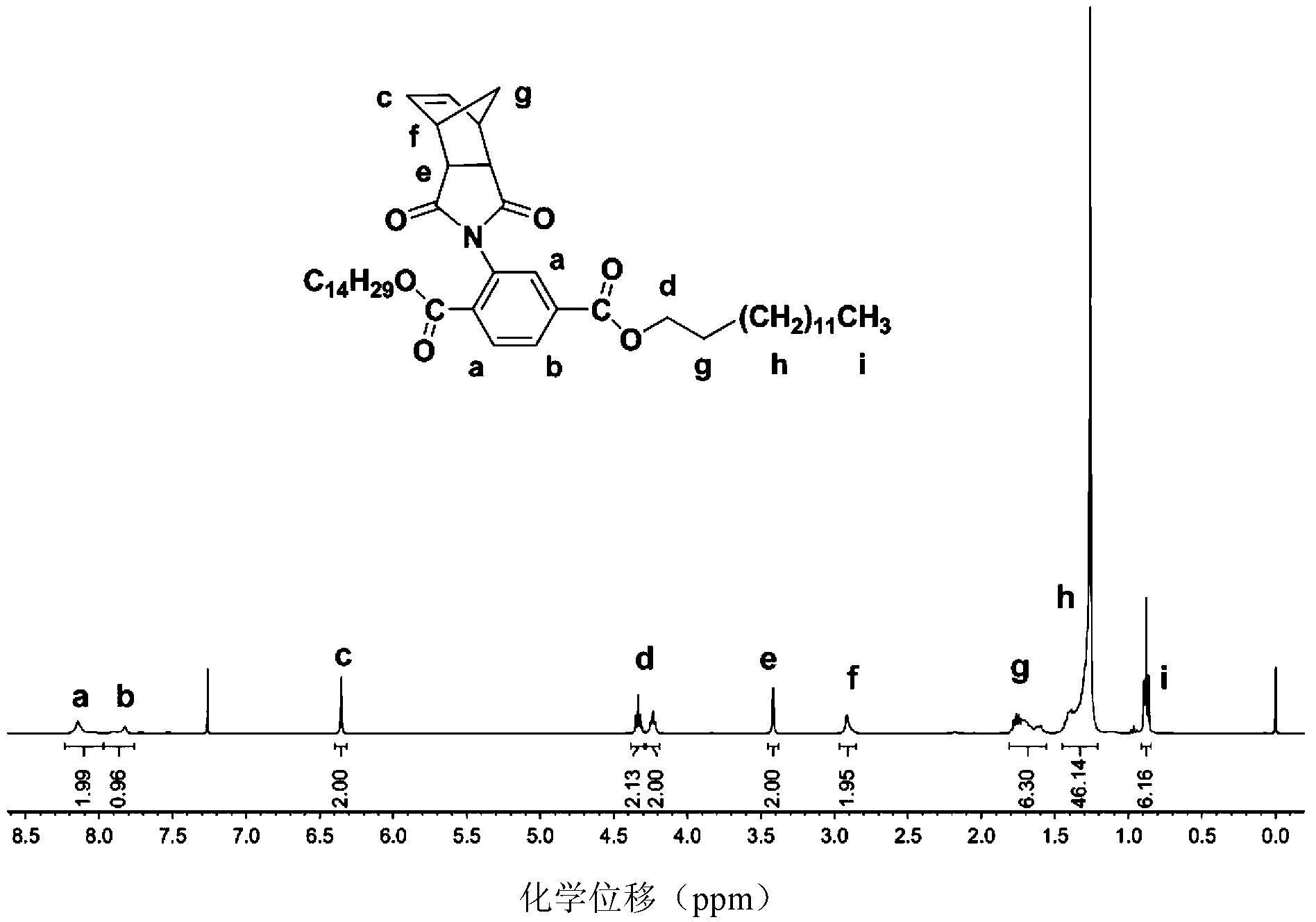
[0042] Embodiment: the preparation of poly-2,5-dicarboxylate phenyl norbornene imide
[0043]The preparation reaction route of poly-2,5-dicarboxylic acid diester phenyl norbornene imide is as follows:
[0044]
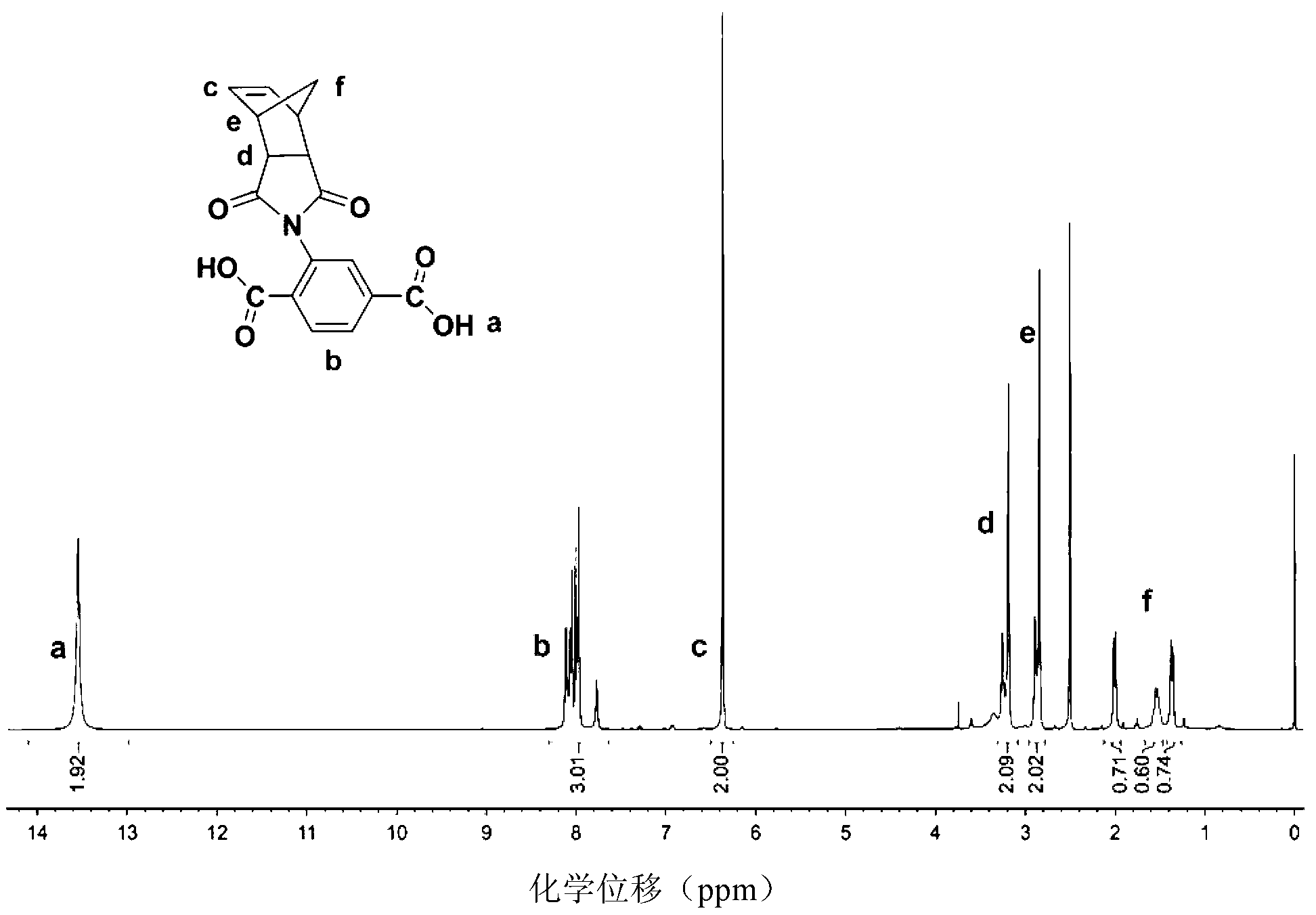
[0045] 1. Synthesis of 2,5-dicarboxylic acid phenyl norbornene imide intermediate:
[0046] Add cis-5-norbornene-exo-2,3-dicarboxylic anhydride (2.00g, 12.2mmol) and glacial acetic acid (30ml) into the three-necked flask, add 2,5- Dicarboxyaniline (2.21g, 12.2mmol) (completely added in 30 minutes), after reacting for 12h, pour the reaction solution into about 100ml of cold water, filter, and dissolve the yellow filter residue in saturated NaHCO 3 Aqueous solution, filter out insoluble matter, acidify the filtrate to pH=2 with hydrochloric acid under vigorous stirring, filter, wash the filter cake with water until neutral, and dry to obtain 3.03 g of white solid. Yield 76%. 1 H NMR (400MHz, DMSO, δ, ppm): 13.53(s, 2H), 7.77-8.11(m, 3H), 6.37(s, 2H), 3.19-3.26(d, 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
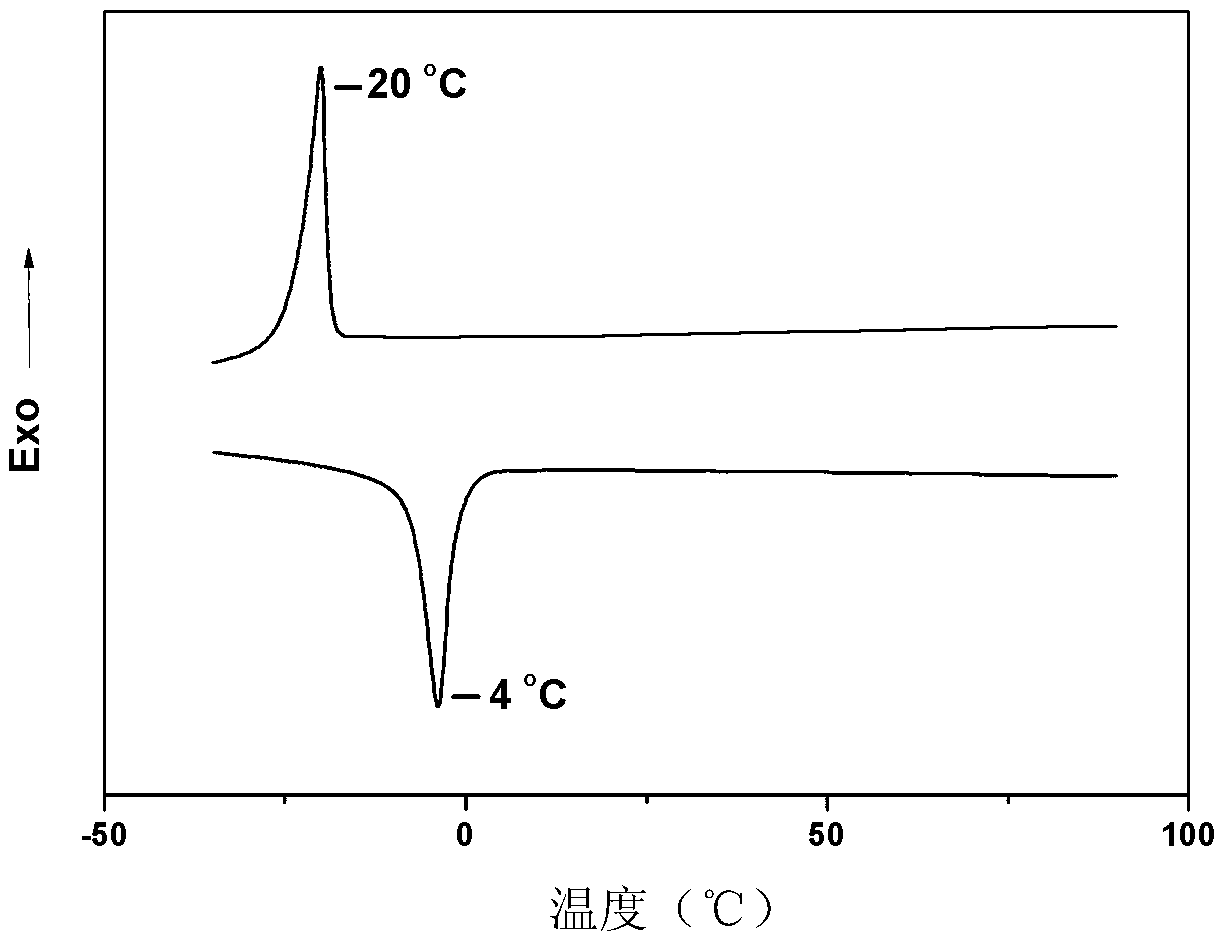
| glass transition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com