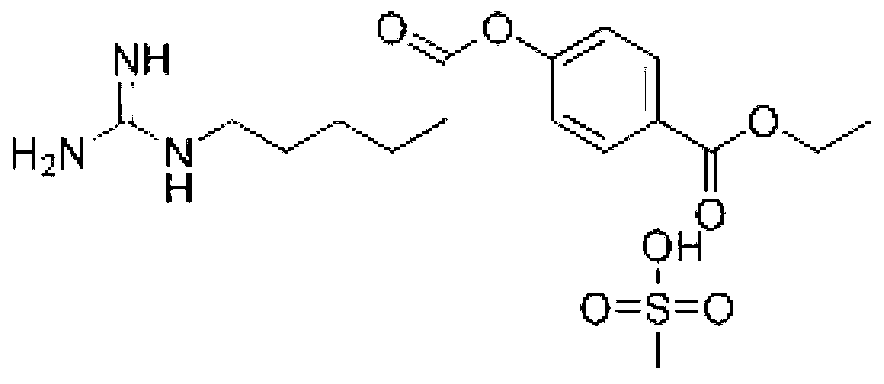
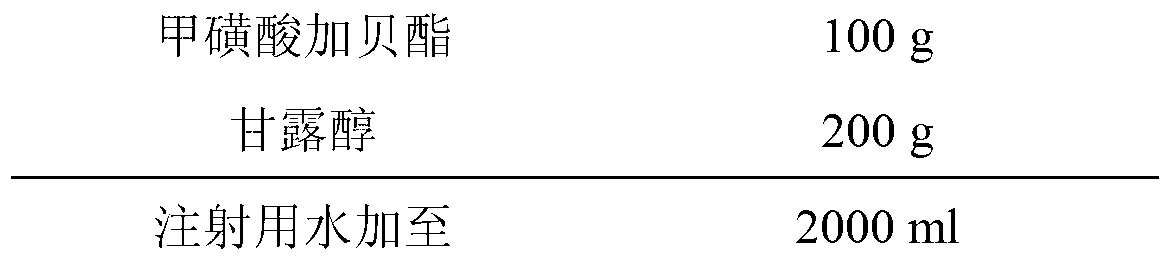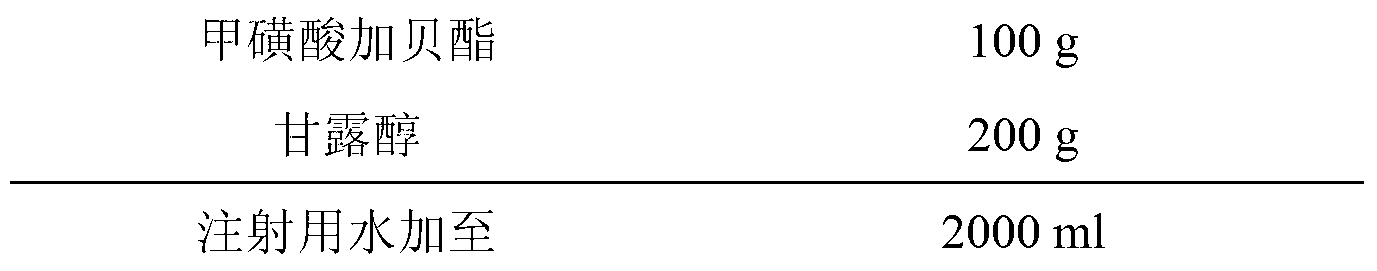Gabexate mesylate composition for injection and preparation method thereof
A technology of gabexate mesylate and its composition, which is applied in the field of medicine, can solve the problems of affecting the efficacy of the product and the decrease of the main drug content, and achieve the effect of eliminating safety risks, simple prescription and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the preparation of gabexate mesylate for injection
[0021] Prescription: (based on 1000 bottles)
[0022]
[0023] Preparation process: (1) Take 200g of mannitol and add it to 2000ml of water for injection. After stirring and dissolving, add 0.1% (w / v) medicinal charcoal, stir well and keep the temperature at about 60°C for 15 minutes. Use 0.45μm Coarse filtration with a microporous membrane to obtain a filtrate. (2) Add 100g of Gabexate mesylate into the mannitol filtrate, dissolve and stir, fine filter with a 0.22μm microporous membrane, replenish water to 2000ml, fill in 7ml vials, 2.0ml per bottle, and place in the freezer In a drying oven, freeze-dry (-40°C, pre-freeze for 4 hours and start vacuuming, control the vacuum between 10 and 30 Pa, raise the temperature to -20°C, keep it for 12 hours, raise the temperature to 0°C, keep it for 2 hours, and raise the temperature to 20 ℃, kept for 4h, and the cold trap temperature was kept at -60℃ during ...
Embodiment 2
[0024] Embodiment 2: the preparation of gabexate mesylate for injection
[0025] Prescription: (based on 1000 bottles)
[0026]
[0027] Preparation process: (1) Take 200g of mannitol and add it to 2000ml of water for injection. After stirring and dissolving, add 0.1% (w / v) medicinal charcoal, stir well and keep the temperature at about 70°C for 20 minutes. Use 0.45μm Coarse filtration with a microporous membrane to obtain a filtrate. (2) Add 100g of Gabexate mesylate into the mannitol filtrate, dissolve and stir, fine filter with a 0.22μm microporous membrane, replenish water to 2000ml, fill in 7ml vials, 2.0ml per bottle, and place in the freezer In a drying oven, freeze-dry (-40°C, pre-freeze for 4 hours and start vacuuming, control the vacuum between 10 and 30 Pa, raise the temperature to -20°C, keep it for 12 hours, raise the temperature to 0°C, keep it for 2 hours, and raise the temperature to 20 ℃, kept for 4h, and the cold trap temperature was kept at -60℃ during ...
Embodiment 3
[0028] Embodiment 3: the preparation of gabexate mesylate for injection
[0029] Prescription: (based on 1000 bottles)
[0030]
[0031] Preparation process: (1) Take 200g of mannitol and add it to 2000ml of water for injection, stir to dissolve, add 0.1% (w / v) medicinal charcoal, stir well and keep the temperature at about 80°C for 10 minutes, then use 0.45μm Coarse filtration with a microporous membrane to obtain a filtrate. (2) Add 100g of Gabexate mesylate into the mannitol filtrate, dissolve and stir, fine filter with a 0.22μm microporous membrane, replenish water to 2000ml, fill in 7ml vials, 2.0ml per bottle, and place in the freezer In a drying oven, freeze-dry (-40°C, pre-freeze for 4 hours and start vacuuming, control the vacuum between 10 and 30 Pa, raise the temperature to -20°C, keep it for 12 hours, raise the temperature to 0°C, keep it for 2 hours, and raise the temperature to 20 ℃, kept for 4h, and the cold trap temperature was kept at -60℃ during the free...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com