Preparation of two novel non-ATP (Adenosine Triphosphate) competitive FGFR1 (Fibroblast Growth Factor Receptor1) inhibitors and anti-tumor activity of two novel non-ATP competitive FGFR1 inhibitors
A tumor and tumor-related technology, applied in the fields of anti-tumor drugs, organic chemistry, drug combination, etc., can solve the problems of loss of pharmacological effects, influence, and large toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
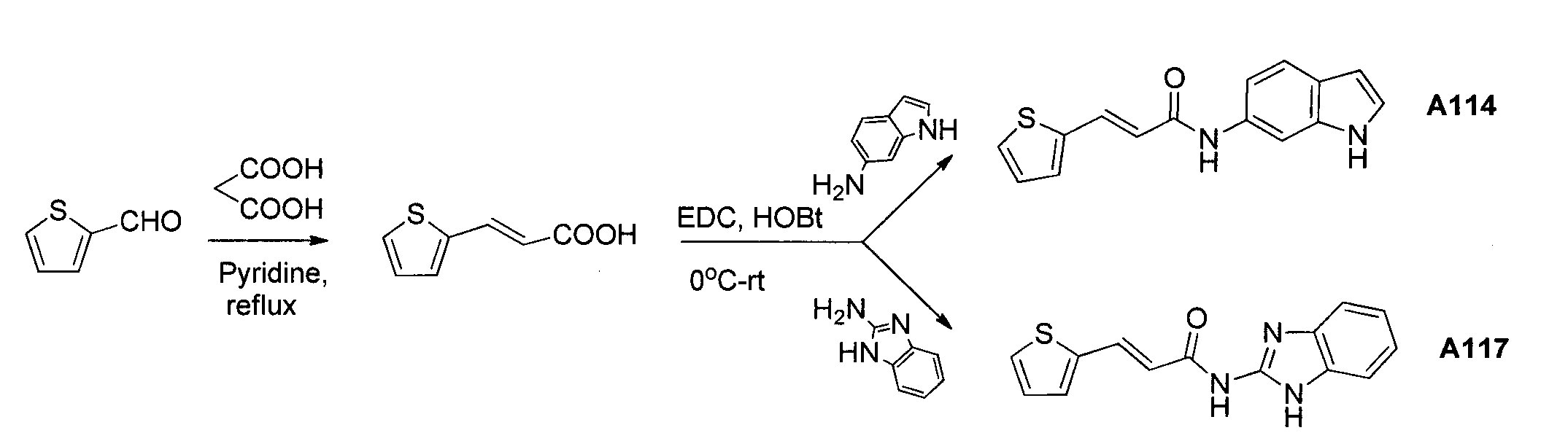
[0024] The preparation of embodiment 1 compound A114, A117:
[0025] Preparation of intermediate thiophene-2-acrylic acid: Add 0.024mol (2.50g) malonic acid, 0.02mol thiophene-2-carbaldehyde and 0.048mol (3.80g) dry pyridine in a 100ml round bottom flask, then add 0.0005 mol (0.047g) aniline, the mixture was heated to reflux at about 95°C, and the reaction process was monitored by TLC. After the reaction of the raw materials was completed, the reaction was terminated. After cooling, 3mol / L aqueous HCl solution was added dropwise to the system until no solids were produced, filtered, Wash with ice water until the filtrate is neutral, recrystallize and dry to obtain thiophene-2-acrylic acid.
[0026] Preparation of compound (E)-N-(1Hindole-6)-(thiophene-2)-acrylamide (A114): Dissolve 0.11g (0.86mmol) of 5-aminoindole in an appropriate amount of In tetrahydrofuran, after stirring for 5min, add 0.16g (0.16mmol) triethylamine dropwise to the system, after 10min, add 0.13g (0.78mmo...
Embodiment 2
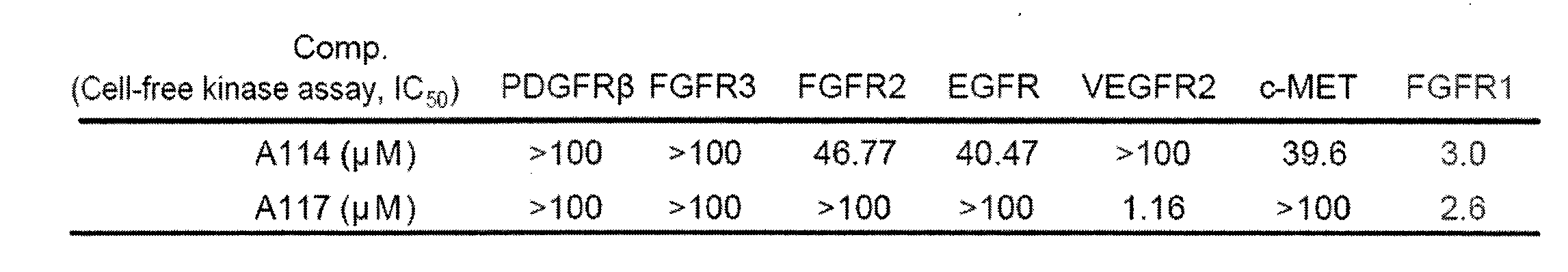
[0030] Example 2 Compound Inhibits FGFR1 Activity and Selectivity in Vitro
[0031]In vitro activity screening of FGFR1 kinase: The method used in the experiment is Caliper Mobility Shift Assay, which is a detection platform based on the mobility detection technology of microfluidic chip technology. Experimental steps: configure 1.25x kinase reaction buffer (62.5mmol / L HEPES, pH7.5; 0.001875% Brij-35; 12.5mmol / LMgCl2; 2.5mM DTT) and kinase reaction termination solution (100mmol / L HEPES, pH7.5 ; 0.015% Brij-35; 0.2% Coating Reagent#3); in 5 μl of 5x concentration compound solution (dissolved in DMSO, diluted 10 times with water), add 10 μl of 2.5x FGFR1 kinase solution (in 1.25x kinase reaction buffer add kinase in solution), incubate at room temperature for 10min, then add 10μl of 2.5x substrate peptide solution (add FAM-labeled peptide and ATP in 1.25x kinase reaction buffer), add 25μl kinase reaction after reacting at 28℃ for a specific time stop solution. Test and collect...
Embodiment 3
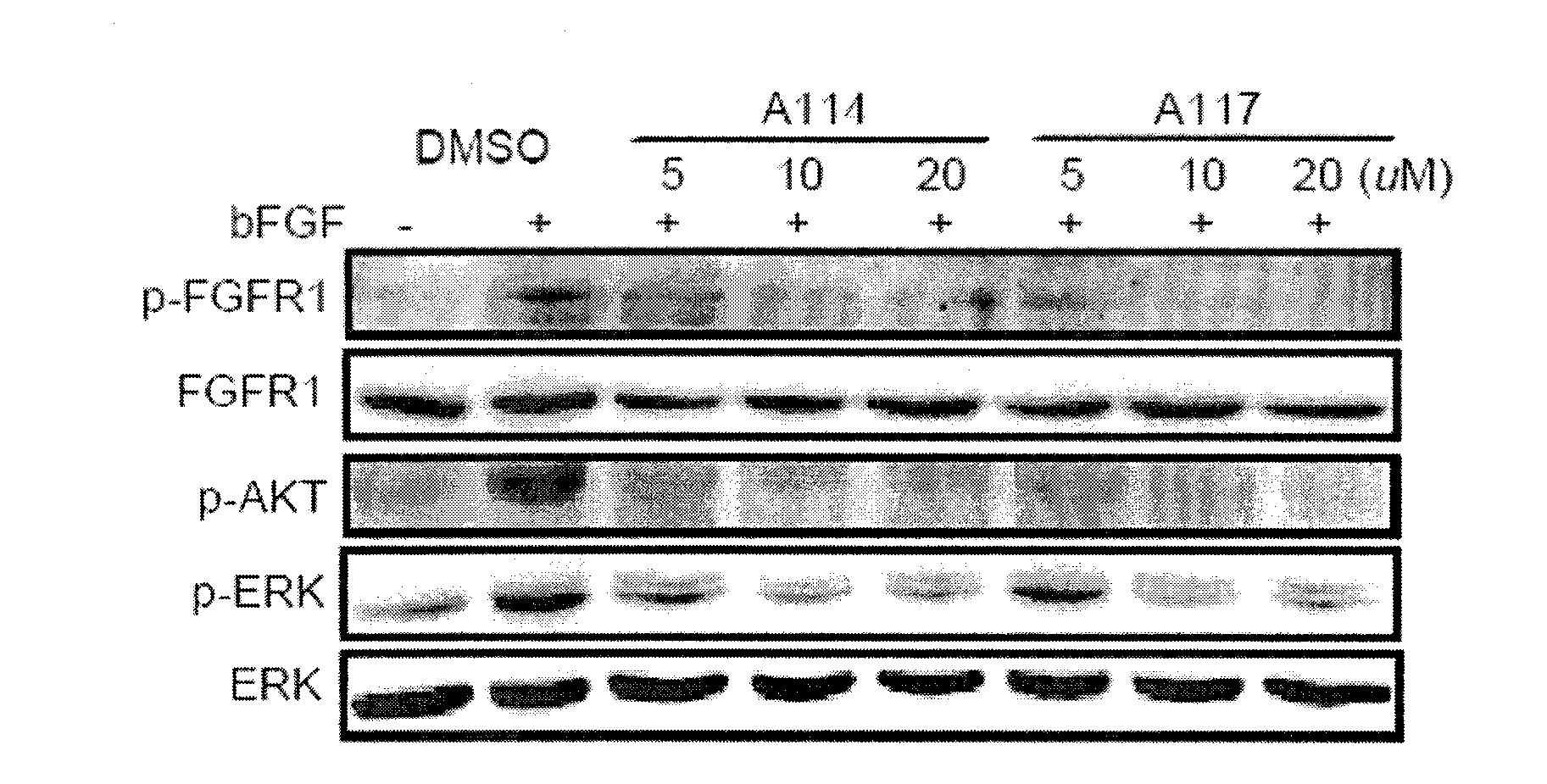
[0034] Antitumor Activity Verification of Compounds at the Cell Level in Example 3
[0035] Compound A114 and compound A117 were formulated into 5, 10 and 20um concentration solutions with DMSO, respectively, and bFGF and bFGF combined with the above-mentioned different concentrations of active compounds were used to treat H460 and MRC-5 cells with high FGFR1 expression, and the anti-inflammatory effects of the two compounds were detected. Tumor proliferation activity; at the same time, total cell protein was extracted, and Western blotting was used to detect the changes in the phosphorylation levels of the pro-proliferation signal molecules FGFR, ERK and AKT in the bFGF / FGFR signaling pathway in the cells. Both compounds have good anti-tumor proliferation activity, and can strongly inhibit p-FGFR1, p-AKT and p-ERK levels at three concentrations of 5, 10 and 20um (see image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com