Scar and tissue fibration resistant oligomeric double-stranded nucleotide medicine and its application
A technology of double-stranded nucleotides and nucleic acid drugs, which can be used in drug combinations, antipyretics, anti-inflammatory agents, etc., and can solve problems such as difficult to achieve
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, preparation and analysis of decoy nucleic acid drug
[0040] Synthesize four of the following thio double-stranded oligomeric decoy nucleic acid drugs: AFODN1: Mix equal amounts of SEQ ID NO.1 and SEQ ID NO.2 in PBS buffer, denature at 94°C for 5 minutes, and anneal to form a double-stranded oligo Polymeric DNA; AFODN2: SEQ ID NO.3 and SEQ ID NO.4 are mixed in equal amounts in PBS buffer, denatured at 94°C for 5 minutes, and then annealed into double-stranded oligomeric DNA; AFODN3: SEQ ID NO.5 and SEQ ID NO.5 Mix equal amounts of ID NO.6 in PBS buffer, and anneal to double-stranded oligomeric DNA after denaturation at 94°C for 5 minutes; AFODN4: mix equal amounts of SEQ ID NO.7 and SEQ ID NO.8 in PBS buffer , after denaturation at 94°C for 5 minutes, annealed into double-stranded oligomeric DNA.
[0041] Scramble negative control sequence sense strand: 5′-GACGCAAGCAGTAGCTATTGCTCAGTCTACCATC-3′
[0042] Antisense strand: 5′-GATGGTAGACTGAGCAATAGCTACTGCTT...
Embodiment 2
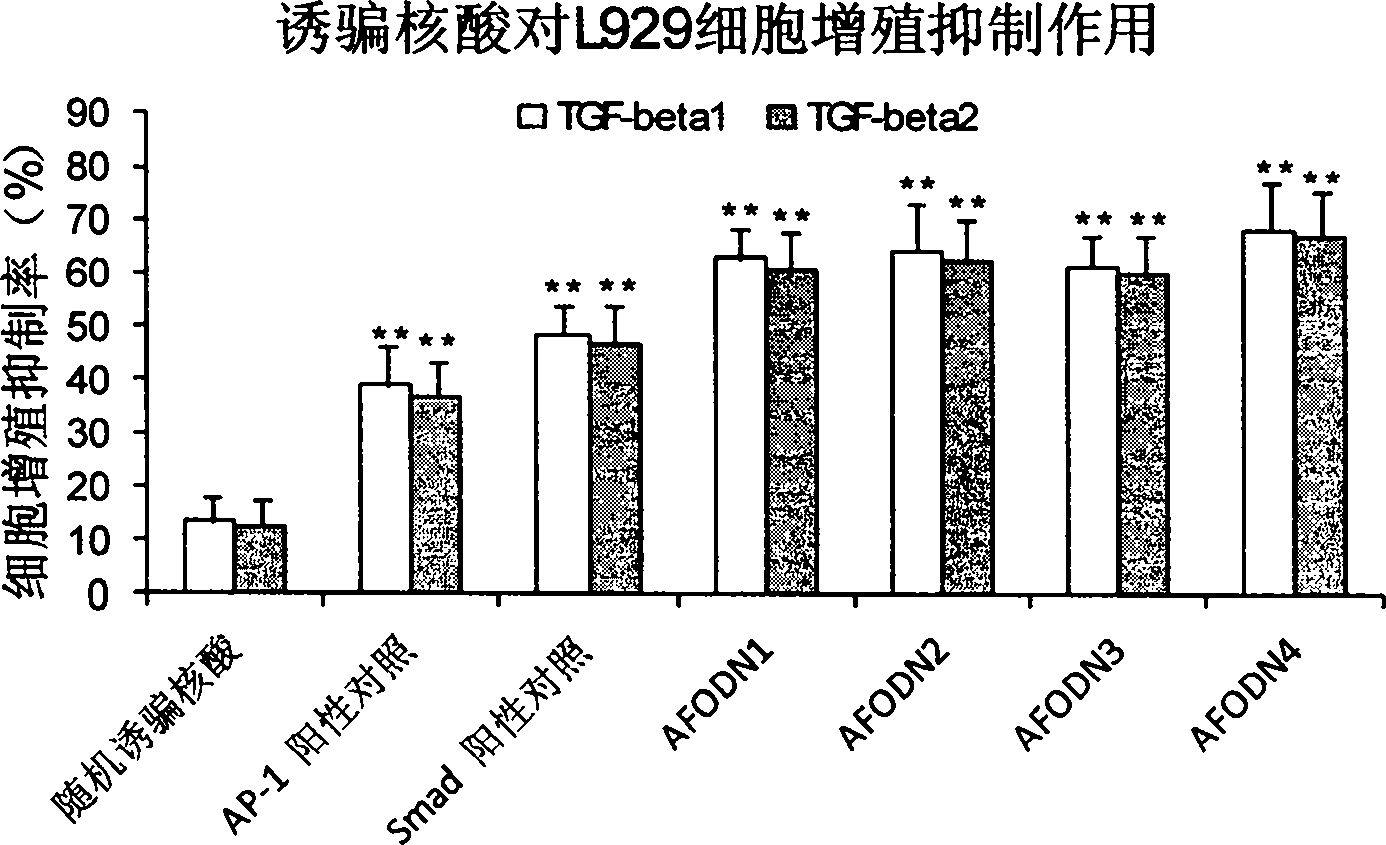
[0048] Example 2. Growth inhibition of L929 fibroblasts by four kinds of AFODN series decoy nucleic acids
[0049] Resuscitate and inoculate L929 fibroblasts in cell culture flasks at 37°C, 5% CO 2 Under certain conditions, culture cells with RPMI 1640 complete culture medium containing 10% FBS; when the cells reach 70%-80% confluence, trypsinize the cells, count the viable cells and adjust the cell concentration to 2×10 5 Cells / ml; Inoculate cells with 100 μL cell suspension per well in a 96-well plate and incubate for 12 hours; then replace with serum-free medium, and randomly divide cells into blank control group, positive control group, negative control group and four AFODN drugs group, and each group was provided with 3 multiple wells, wherein only 0.6 μl / well liposome was added to the blank control group, and 0.6 μl / well liposome and different decoy nucleic acids with a final concentration of 100 nM were added to the rest; after 5 hours of transfection, Replace it wit...
Embodiment 3
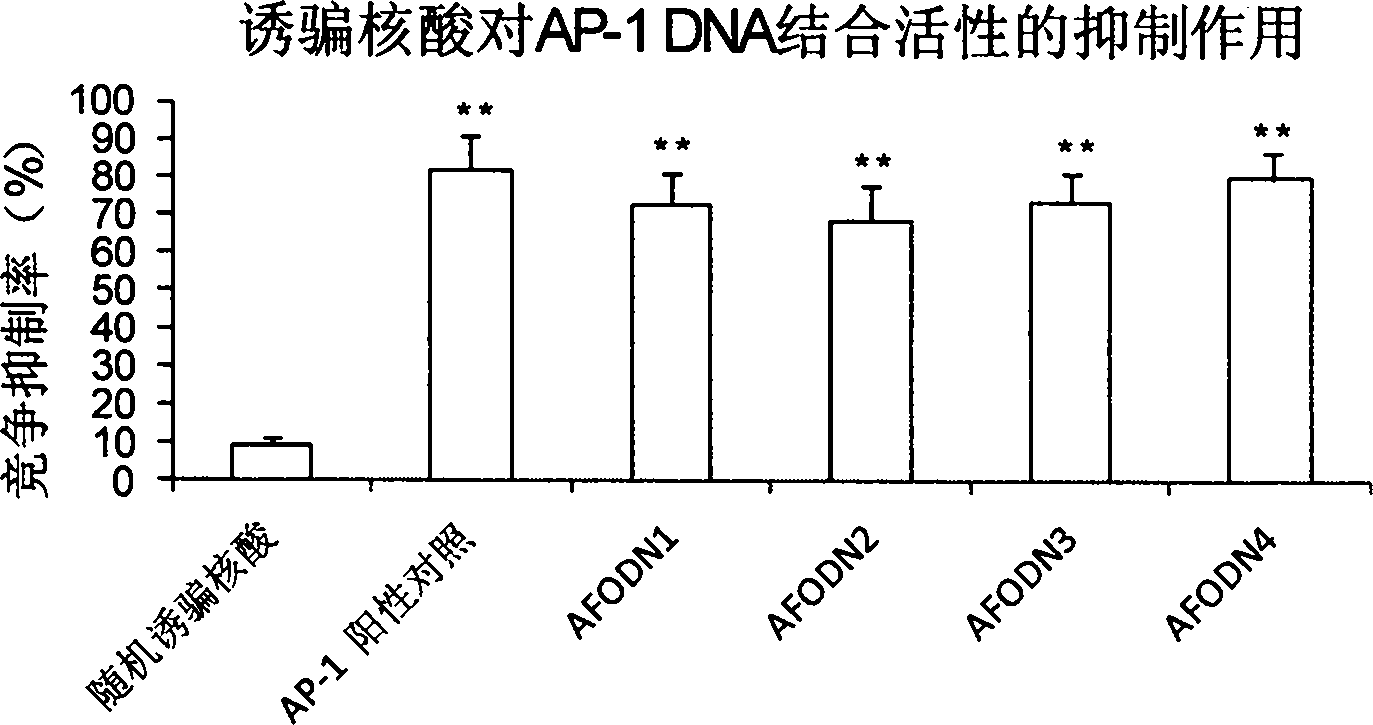
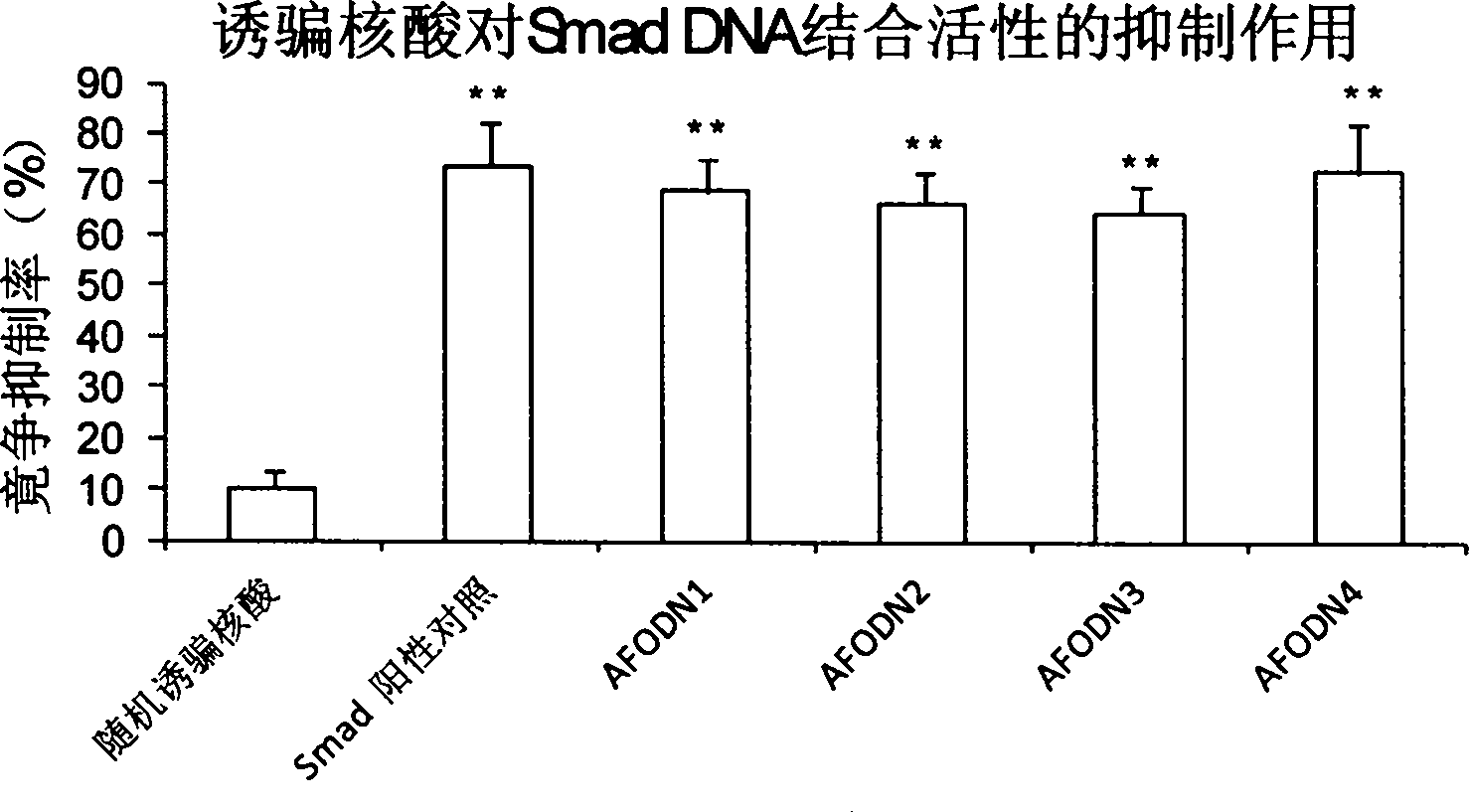
[0051] Example 3, ELISA method to detect the competition of decoy nucleic acid for the binding of AP-1 and Smad to its cis-acting elements Inhibition experiment
[0052] Dilute 10 pmol of AFODN series drugs in 30 μl of complete binding buffer, and add 30 μl per hole to a 96-well enzyme-linked plate coated with Ap-1 or Smad cis-acting elements (purchased from TransAM from Active Motif, USA). TM Transcription factor assay kits); then dilute 5 μg TPA-induced K-562 nuclear extract (for Smad, use TGF-β1-induced L929 fibroblast nuclear extract) in 20 μl complete binding buffer, and then add Mix well in a 96-well ELISA plate and incubate at room temperature for 1 h. Note that the detection of various samples was carried out in 3 duplicate wells, and the experimental groups were as follows: blank negative control only added 50 μl complete binding buffer without AFODN drug and cell nucleus extract; blank positive control was added without AFODN drug, only containing 50 μl of comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com