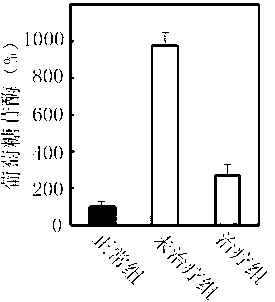Pharmaceutical composition for treating diabetes and indications thereof
A technology for indications and diabetes, which is applied in the field of pharmaceutical compositions for the treatment of diabetes and its indications, can solve the problems of insignificant curative effect of the indications and the like, and achieve the effect of inhibiting blood sugar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the establishment of diabetes model
[0023] In the embodiment of the present invention, the STZ (streptozotocin) method was used to induce type I diabetic rats.
[0024] Preparation of type I diabetes rat model: Take SD rats with a body weight of about 160g, fast for 24 hours, and inject intraperitoneally with a dose of 60 mg STZ / kg body weight (STZ is prepared with 0.05 M citric acid solution (pH4.5) ); 24, 48, and 72 hours later, the blood glucose was measured by docking the tail, and the rats that exceeded 250 mg / dL for 3 consecutive days were determined as diabetic rats; the unsuccessful rats were re-injected with the same amount after 24, 48 hours Rats with blood glucose lower than 250 mg / dL will be excluded, and the rat model thus prepared is a type I diabetes model.
[0025] Preparation of type II diabetes rat model: After feeding the rats with high fat for 4 weeks, a single dose of 100 mg STZ / kg body weight was injected intraperitoneally; Rats w...
experiment example 2
[0026] Experimental Example 2: Preparation and use of drug extraction and drug combination capsules
[0027] Preparation of extracts from turmeric, rhubarb, turmeric, aloe vera, and knotweed: grind appropriate amount of medicinal materials, extract several times with ethanol reflux, filter and combine filtrates, freeze-dry to dryness. The extracts of various medicinal materials are mixed by equal weight to form a medicinal material extraction mixture, and folic acid and coenzyme Q10 are added, and starch is used as a filler to make capsules. The dosage range of each component of each capsule is: the above medicinal material extraction mixture 2.5mg, folic acid 1mg And coenzyme Q10 50mg, processed into capsules with starch as filler. When feeding animals, each capsule is dissolved in 500 ml of feeding water.
Embodiment 3
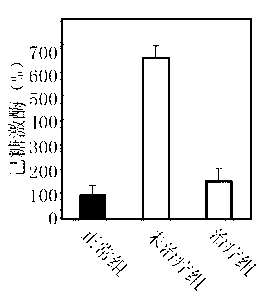
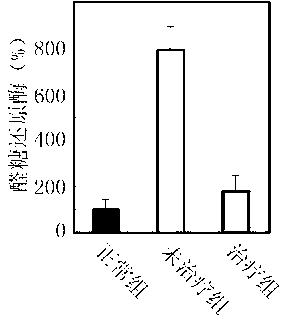
[0028] Example 3: Inhibition of the combined formulation on α-glucosidase, hexokinase, and aldose reductase
[0029] For the type I diabetes rat model, after being fed with water containing the above drug combination for one month, blood samples were taken from the tail vein, and the activities of α-glucosidase, hexokinase and aldose reductase were measured respectively.
[0030] Referring to the method of Matsui et al., the activity of α-glucosidase was determined with pNP-G as substrate (J. Agri. Food Chem. 2001 (49): 1948-1951), and α-glucosidase (0.1U / ml ) After incubating in a water bath at 37°C for 10 minutes, the substrate pNP-G (2 mM, 0.1ml) was added to initiate the reaction, and after 20 minutes, 1 mL of 0.5 M Na 2 CO 3 Stop the reaction. Finally, measure the light absorption value of p-nitrobenzene released from pNP-G under the action of enzyme at 405nm, and calculate the inhibition efficiency.
[0031] The determination of hexokinase activity is described in re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com