Catalyst for synthesising methanol by hydrogenation of carbon dioxide as well as preparation method and application thereof
A technology for synthesizing methanol and carbon dioxide, applied in the direction of metal/metal oxide/metal hydroxide catalysts, preparation of hydroxyl compounds, preparation of organic compounds, etc., to achieve good performance repeatability, improve conversion rate and selectivity, and simple preparation method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
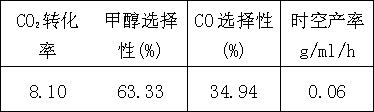
[0017] Embodiment 1: take following metal nitrate as precursor, prepare mixed solution, its metal atom molar ratio is La10Cu7Zn3, citric acid molar weight is 2 times of metal element total amount. Stir to remove water at 80°C, raise the temperature to 120°C after it becomes gelatinous, further dehydrate to form a gel, and ignite. The burned powder was then calcined at 1000°C for 4 hours to obtain the composition La 10 Cu 7 Zn 3 o x catalyst. Sieve the calcined catalyst tablet and crush it to 40-60 mesh for reaction evaluation.
[0018] CO 2 +H 2 The reaction was carried out in a fixed-bed reactor. Before the reaction, the catalyst was reduced with high-purity hydrogen at 330°C for 6 hours, and then switched to CO 2 +H 2 The mixed gas was reacted, and the reaction conditions were as follows: P=5.0 MPa, T=250 ℃, GHSV=3600 h -1 , n(H 2 ) / n(CO 2 ) molar ratio=3, the liquid phase product was collected in an ice-water bath, and the product composition was analyzed by gas ...
Embodiment 2
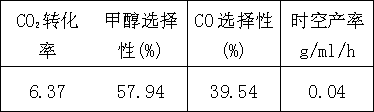
[0020] Example 2: Use the following metal nitrates as precursors to prepare a mixed solution, the molar ratio of metal atoms is La4Y2Cr2Cu6Zn3, and the molar weight of citric acid is 1.5 times the total amount of metal elements. Stir at 60°C to remove water, raise the temperature to 150°C after it becomes gelatinous, further dehydrate to form a gel, and ignite. The burned powder is then calcined at 800°C for 4 hours to obtain the composition La 4 Y 2 Cr 2 Cu 6 Zn 3 o x catalyst. The calcined catalyst was pressed into tablets and sieved, crushed to 40-60 mesh for reaction evaluation.
[0021] Reaction conditions: P=2.0 MPa, T=230 ℃, GHSV=2000 h -1 , n(H 2 ) / n(CO 2 ) molar ratio=2, the obtained results are as follows:
[0022]
Embodiment 3
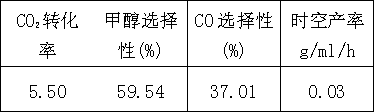
[0023] Example 3: Using the following metal nitrates as precursors, a mixed solution was prepared, the molar ratio of metal atoms was La3Ce2Cu7Zn3, and the molar amount of citric acid was 1.2 times the total amount of metal elements. Stir to remove water at 80°C, raise the temperature to 170°C after it becomes gelatinous, further dehydrate to form a gel, and ignite. The burned powder is then calcined at 500°C for 8 hours to obtain the composition La 3 Ce 2 Cu 7 Zn 3 o x catalyst. The calcined catalyst was pressed into tablets and sieved, crushed to 40-60 mesh for reaction evaluation.
[0024] Reaction conditions P=6.0 MPa, T=350 ℃, GHSV=5000 h -1 , n(H 2 ) / n(CO 2 ) molar ratio=4, the obtained results are as follows:
[0025]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com