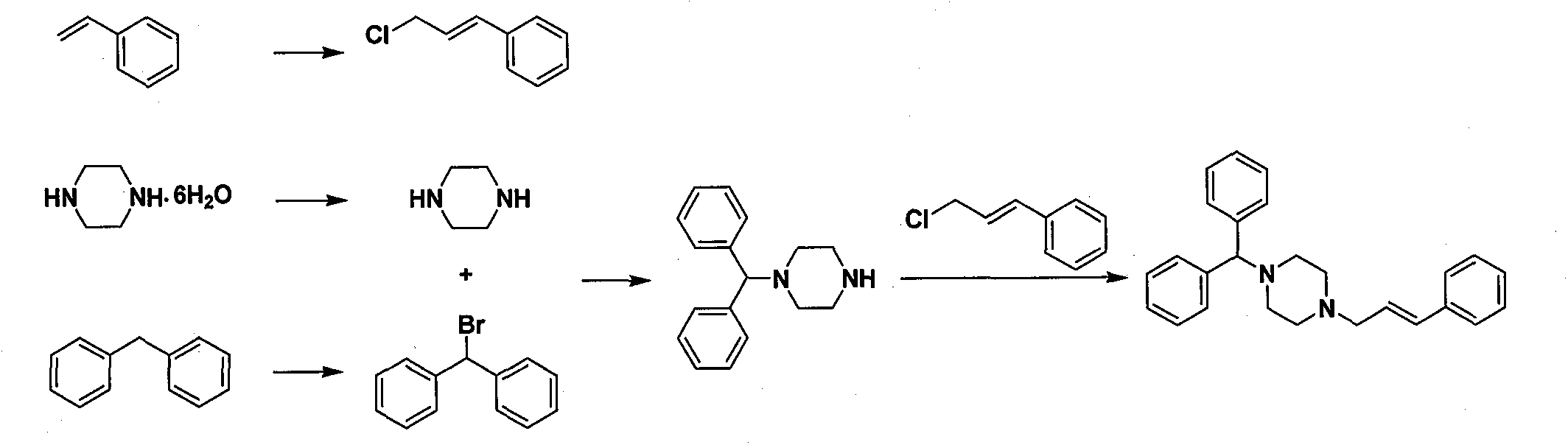
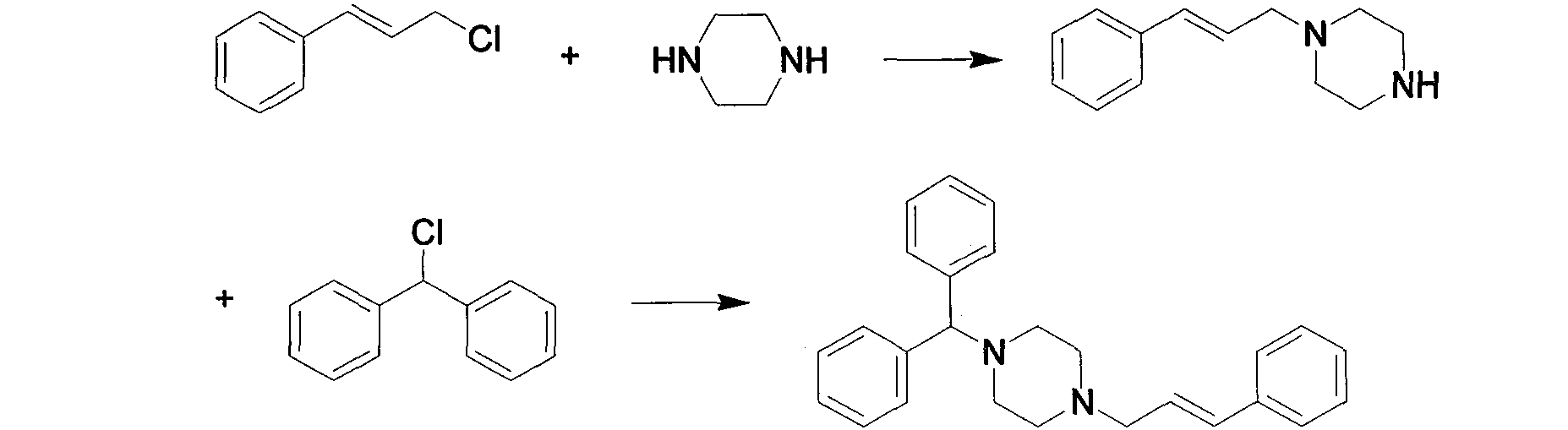
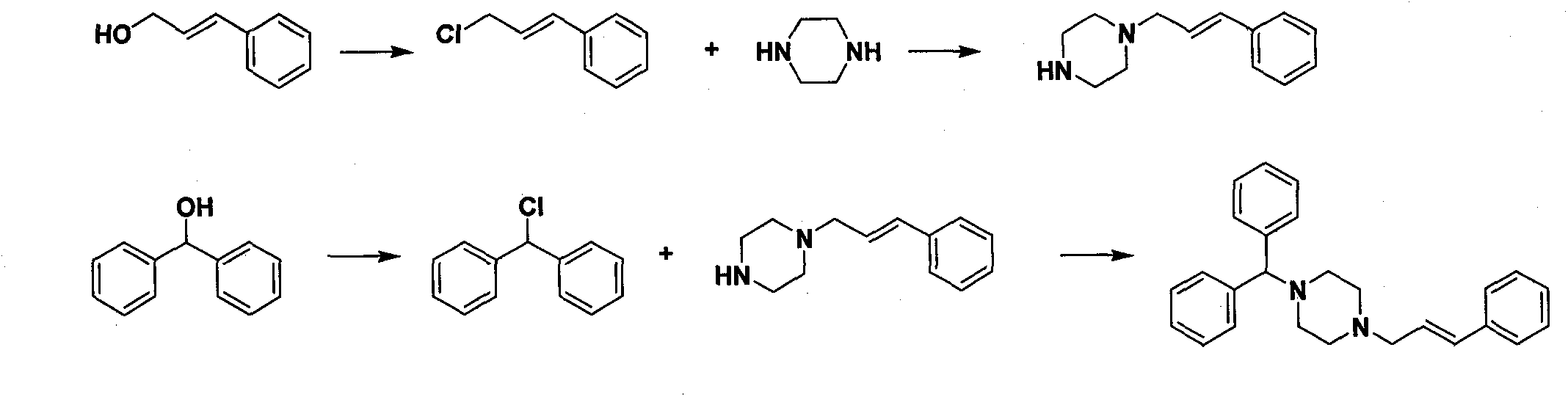
New synthesis method of cinnarizine
A technology of cinnarizine and anhydrous piperazine, applied in the field of pharmaceutical preparation, can solve the problems of many by-products, difficult industrialized large-scale production, unfavorable purification and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]Add 11mol / L zinc chloride solution (15ml, 0.165mol) and benzene (30ml, 0.155mol) into a 250ml three-necked flask, stir and raise the temperature until benzene refluxes, start to add 9.0g of benzyl chloride dropwise, and react for 4 hours after the dripping is completed. Set aside, separate the zinc chloride solution in the lower layer, add 180ml of water to the upper layer, stir for 5 minutes, let stand for 10 minutes, collect the organic phase, and rotary evaporate to dryness to obtain 26.0 g of diphenylmethane product, with a yield of 96% and a GC purity of 98%.
Embodiment 2
[0046] Add 11mol / L ferric chloride solution (15ml, 0.165mol) and benzene (30ml, 0.155mol) into a 250ml three-necked flask, stir and heat up to reflux of benzene, start to add 9.0g of benzyl chloride dropwise, and react for 4h after the dropwise completion. After standing still, separate the lower layer of zinc chloride solution, add 180ml of water to the upper layer, stir for 5 minutes, let stand for 10 minutes, collect the organic phase, and rotary evaporate to dryness to obtain 9.5g of diphenylmethane product, the yield is 35%, and the GC purity is 96%.
Embodiment 3
[0048] Add 11mol / L aluminum chloride solution (15ml, 0.165mol) and benzene (30ml, 0.155mol) into a 250ml three-necked flask, stir and raise the temperature to reflux of benzene, start to add 9.0g of benzyl chloride dropwise, and react for 4h after the dropwise completion. After standing still, separate the zinc chloride solution in the lower layer, add 180ml of water to the upper layer, stir for 5 minutes, let stand for 10 minutes, collect the organic phase, and rotary evaporate to dryness to obtain 20.6g of diphenylmethane product, with a yield of 76% and a GC purity of 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com